C8511
Cathepsin C from bovine spleen
Type X, lyophilized powder, ≥5 units/mg protein
Synonym(e):
Dipeptidyl aminopeptidase, Dipeptidyl peptidase I
About This Item
Empfohlene Produkte
Biologische Quelle
bovine spleen
Typ
Type X
Assay
>25% protein (biuret)
Form
lyophilized powder
Spezifische Aktivität
≥5 units/mg protein
Zusammensetzung
Protein, ≥25% biuret
Hersteller/Markenname
Sigma-Aldrich
Lagerbedingungen
OK to freeze (Unstable. Keep frozen)
Konzentration
≥5 unit/mg protein
Methode(n)
activity assay: suitable
Eignung
suitable for molecular biology
Anwendung(en)
life science and biopharma
Versandbedingung
dry ice
Lagertemp.
−20°C
Angaben zum Gen
cow ... CTSC(352958)
Allgemeine Beschreibung
Dipeptidyl peptidase I (DPPI), also known as cathepsin C, is an abundant lysosomal cysteine protease from the papain superfamily with a molecular weight of approximately 200 kDa. It is widely expressed in a variety of mammalian tissues, with the highest levels found in the lungs, kidneys, liver, and spleen, and relatively lower levels in the brain.
DPPI is the only member of its family that is functional as a tetramer, consisting of four identical subunits, each composed of an N-terminal fragment, a heavy chain, and a light chain. It is identified as one of the multifaceted protease-processing machines, having been shown to function beyond its role as a non-specific lysosomal protease.
Anwendung
Biochem./physiol. Wirkung
Cat C participates in neutrophil recruitment and production of chemokines and cytokines in many inflammatory diseases. Cathepsin C plays a crucial role as an essential enzyme in activating granule serine proteases in cytotoxic T lymphocytes, natural killer cells (granzymes A and B), mast cells (chymase and tryptase), and neutrophils (cathepsin G, proteinase 3, and elastase).
Vorsicht
Einheitendefinition
Physikalische Form
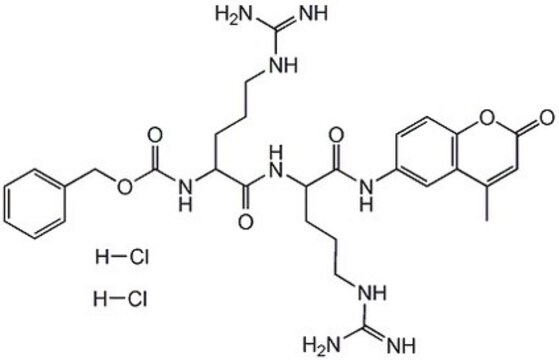
Substrat
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.