B6804
Monoclonal Anti-Bacterial Alkaline Phosphatase (BAP, PhoA) antibody produced in mouse
clone BAP-77, ascites fluid
Synonym(e):
Monoclonal Anti-Phosphatase, Alkaline, bacterial
About This Item
Empfohlene Produkte
Biologische Quelle
mouse
Qualitätsniveau
Konjugat
alkaline phosphatase conjugate
Antikörperform
ascites fluid
Antikörper-Produkttyp
primary antibodies
Klon
BAP-77, monoclonal
Mol-Gew.
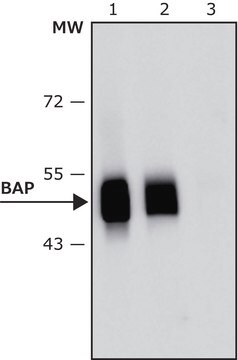
antigen ~50 kDa
Speziesreaktivität
bacteria
Methode(n)
indirect ELISA: suitable
western blot: 1:20,000 using purified Escherichia coli BAP
Isotyp
IgG1
Versandbedingung
dry ice
Lagertemp.
−20°C
Posttranslationale Modifikation Target
unmodified
Allgemeine Beschreibung
Immunogen
Anwendung
Biochem./physiol. Wirkung
Haftungsausschluss
Sie haben nicht das passende Produkt gefunden?
Probieren Sie unser Produkt-Auswahlhilfe. aus.
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.