Wichtige Dokumente
06863
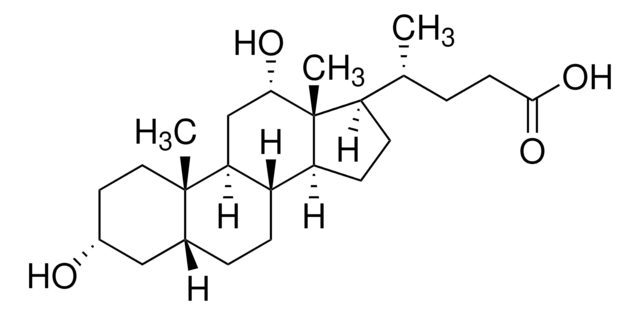
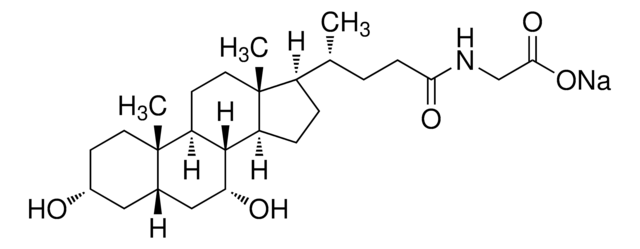
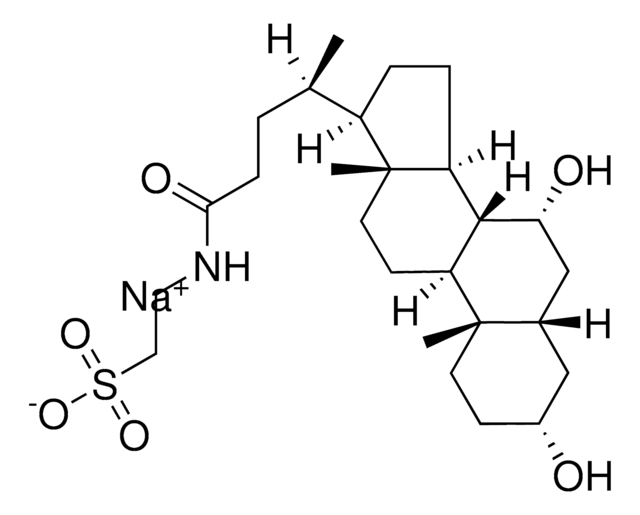
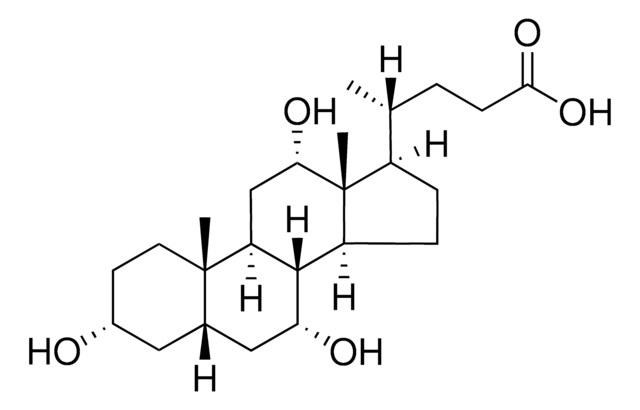
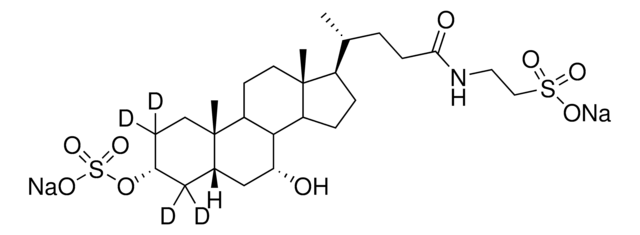
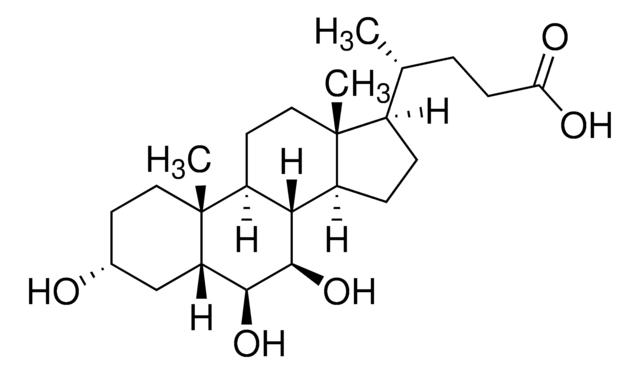
Glykoursodeoxycholsäure
≥96.0% (TLC)
Synonym(e):
N-(3α,7β-Dihydroxy-5β-cholan-24-oyl)-glycin, N-[(3α,5β,7β)-3,7-Dihydroxy-24-oxocholan-24-yl]glycin, Glycylursodeoxycholsäure, Ursodeoxycholylglycin
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Assay
≥96.0% (TLC)
Form
powder
Funktionelle Gruppe
carboxylic acid
SMILES String
[H][C@@]12[C@]([C@](CC[C@@H](O)C3)(C)[C@]3([H])C[C@@H]2O)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)CCC(NCC(O)=O)=O
InChI
1S/C26H43NO5/c1-15(4-7-22(30)27-14-23(31)32)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)29/h15-21,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16+,17-,18-,19+,20+,21+,24+,25+,26-/m1/s1
InChIKey
GHCZAUBVMUEKKP-XROMFQGDSA-N
Anwendung
- Glycoursodeoxycholic Acid Alleviates Arterial Thrombosis via Suppressing Diacylglycerol Kinases Activity in Platelet.: Highlights the therapeutic potential of Glycoursodeoxycholic acid in alleviating arterial thrombosis by inhibiting diacylglycerol kinase activity in platelets (Yang et al., 2024).
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Protokolle
This method is particularly useful in research into the role of individual bile acids as signaling molecules; suitable for clinical laboratories to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.