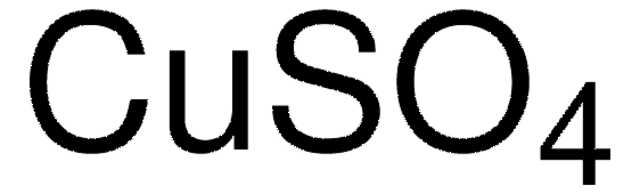Wichtige Dokumente
209198
Kupfer(II)-sulfat Pentahydrat
ACS reagent, ≥98.0%
Synonym(e):
Schwefelsäure Kupfer(II)-salz (1:1) Pentahydrat, Vitriolblau
About This Item
Empfohlene Produkte
Qualität
ACS reagent
Qualitätsniveau
Dampfdruck
7.3 mmHg ( 25 °C)
Assay
≥98.0%
98.0-102.0% (ACS specification)
Form
crystals
Eignung der Reaktion
reaction type: click chemistry
reagent type: catalyst
core: copper
pH-Wert
3.5-4.5 (20 °C, 50 g/L)
mp (Schmelzpunkt)
110 °C (dec.) (lit.)
Anionenspuren
chloride (Cl-): ≤0.001%
Kationenspuren
Ca: ≤0.005%
Fe: ≤0.003%
K: ≤0.01%
Na: ≤0.02%
Ni: ≤0.005%
SMILES String
O.O.O.O.O.[Cu++].[O-]S([O-])(=O)=O
InChI
1S/Cu.H2O4S.5H2O/c;1-5(2,3)4;;;;;/h;(H2,1,2,3,4);5*1H2/q+2;;;;;;/p-2
InChIKey
JZCCFEFSEZPSOG-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- In acetylation of alcohols and phenols in the presence of acetic anhydride.
- To synthesize various ynamides via intramolecular coupling of amides with alkynyl bromides.
It can also be used as a template in the synthesis of copper(II) ion-imprinted polymers using vinylpyridine and methacrylic acid as functional monomers. This polymer is used to quantify Cu2+ ion by using flame atomic absorption spectroscopy.
Leistungsmerkmale und Vorteile
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
Lagerklassenschlüssel
13 - Non Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.