Wichtige Dokumente
Y0000109
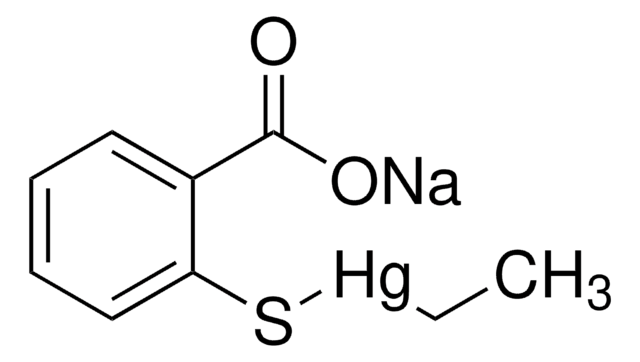
Thiomersal
European Pharmacopoeia (EP) Reference Standard
Synonym(e):
2-(Ethylmercurimercapto)-benzoesäure Natriumsalz, Merthiolate® Natriumsalz, Natriumethylmercurithiosalicylat, Quecksilber-([o-carboxyphenyl]thio)-ethyl Natriumsalz
About This Item
Empfohlene Produkte
Qualität
pharmaceutical primary standard
API-Familie
thimerosal
Hersteller/Markenname
EDQM
mp (Schmelzpunkt)
234-237 °C (dec.) (lit.)
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-8°C
SMILES String
[Na+].CC[Hg]Sc1ccccc1C([O-])=O
InChI
1S/C7H6O2S.C2H5.Hg.Na/c8-7(9)5-3-1-2-4-6(5)10;1-2;;/h1-4,10H,(H,8,9);1H2,2H3;;/q;;2*+1/p-2
InChIKey
RTKIYNMVFMVABJ-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
For further information and support please go to the website of the issuing Pharmacopoeia.
Anwendung
Biochem./physiol. Wirkung
Verpackung
Sonstige Hinweise
Haftungsausschluss
The product is not intended for use as a biocide under global biocide regulations, including but not limited to US EPA′s Federal Insecticide Fungicide and Rodenticide Act , European Biocidal Products Regulation, Canada′s Pest Management Regulatory Agency, Turkey′s Biocidal Products Regulation, Korea′s Consumer Chemical Products and Biocide Safety Management Act (K-BPR) and others.

Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2 Oral
Zielorgane
Kidney
Lagerklassenschlüssel
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Zulassungslistungen
Zulassungslistungen werden hauptsächlich für chemische Produkte erstellt. Für nicht-chemische Produkte können hier nur begrenzte Angaben gemacht werden. Kein Eintrag bedeutet, dass keine der Komponenten gelistet ist. Es liegt in der Verantwortung des Benutzers, die sichere und legale Verwendung des Produkts zu gewährleisten.
EU REACH Annex XVII (Restriction List)
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.