71210
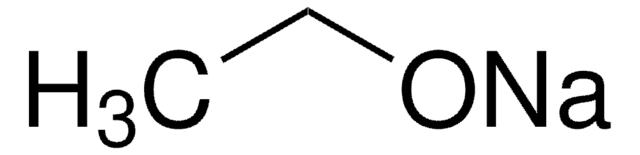
Natriumethoxid
technical, ≥95% (T)
Synonym(e):
Natriumethylat
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
CH3CH2ONa
CAS-Nummer:
Molekulargewicht:
68.05
Beilstein:
3593646
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.21
Empfohlene Produkte
Dampfdichte
1.6 (vs air)
Qualitätsniveau
Dampfdruck
<0.1 mmHg ( 20 °C)
Qualität
technical
Assay
≥95% (T)
Form
powder
Verunreinigungen
~2% Na2CO3 and NaOH
SMILES String
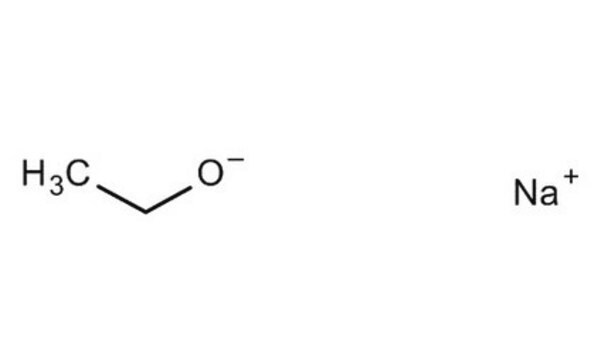
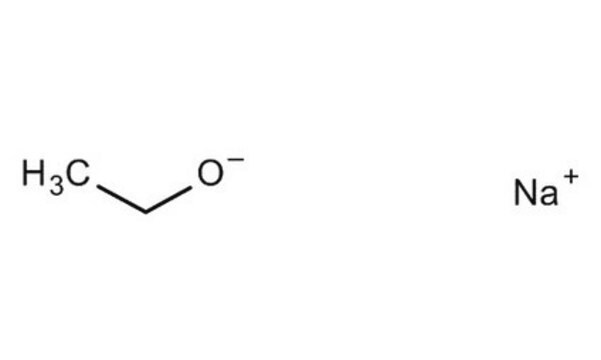
[Na+].CC[O-]
InChI
1S/C2H5O.Na/c1-2-3;/h2H2,1H3;/q-1;+1
InChIKey
QDRKDTQENPPHOJ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Sodium ethoxide (Sodium ethylate) is a sodium alkoxide. It has been synthesized by reacting sodium with ethanol. It undergoes decomposition in the presence of water to afford ethanol and sodium hydroxide. It is widely employed as a strong base in organic synthesis studies.
Sodium ethoxide is an alkoxide salt mainly used as a strong base in organic reactions such as deprotonation, dehydration and dehalogenation.
Anwendung
Sodium ethoxide may be used as a base for the palladium catalyzed cross-coupling of aryl halides and alkenylboranes to synthesize arylated (E)-alkenes.
Sodium ethoxide may be used for the preparation of tricarbonylchloro(glycinato)ruthenium(II) (CORM-3).
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1A
Zusätzliche Gefahrenhinweise
Lagerklassenschlüssel
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flammpunkt (°F)
86.0 °F - closed cup
Flammpunkt (°C)
30 °C - closed cup
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Whitaker KS and Whitaker DT
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst.
Miyaura N & Suzuki, A.
Journal of the Chemical Society. Chemical Communications, 19, 866-867 (1979)
James E Clark et al.
Circulation research, 93(2), e2-e8 (2003-07-05)
Carbon monoxide, which is generated in mammals during the degradation of heme by the enzyme heme oxygenase, is an important signaling mediator. Transition metal carbonyls have been recently shown to function as carbon monoxide-releasing molecules (CO-RMs) and to elicit distinct
Eagleson M.
Concise Encyclopedia Chemistry, 997-997 (1994)
Ali Reza Harifi-Mood et al.
Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques, 7(1), 88-91 (2004-05-18)
Variable-Temperature Kinetics has been used to obtain the rate constants of the reaction at various temperatures during one kinetic run. Pseudo-first-order rate constants for the transesterification of procaine with aliphatic alcohols ethanol, n-propanol and tert-butanol were obtained by the fluorescence
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.