HCMBMAG-22K
MILLIPLEX® Human Cancer/ Metastasis Biomarker Magnetic Bead Panel - Cancer Multiplex Assay
Circulating Cancer and Metastasis Biomarker Bead-Based Multiplex Assays using the Luminex technology enables the simultaneous analysis of multiple oncology biomarkers in various tumor types in human serum, plasma and cell culture samples.
About This Item
Empfohlene Produkte
Qualitätsniveau
Speziesreaktivität
human
Hersteller/Markenname
Milliplex®
assay range
accuracy: 70-105%
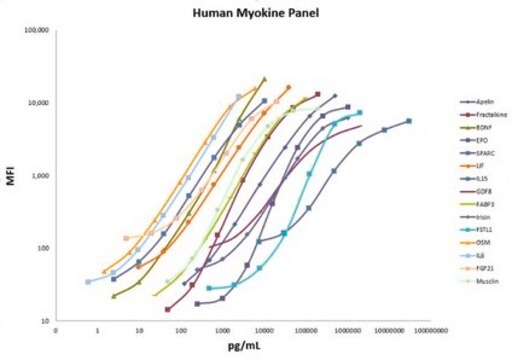
standard curve range: 0,019-80 ng/mL
(TRAP5)
standard curve range: 0,048-20 ng/mL
(YKL40)
standard curve range: 0.002-10 ng/mL
(GDF15)
standard curve range: 0.007-30 ng/mL
(DKK-1)
standard curve range: 0.007-30 ng/mL
(OPG)
standard curve range: 0.036-150 ng/mL
(NSE)
standard curve range: 0.195-800 ng/mL
(Periostin)
standard curve range: 0.976-4,00 0 ng/mL
(Osteonectin (SPARC))
inter-assay cv: <16%
intra-assay cv: <10%
Methode(n)
multiplexing: suitable
Nachweisverfahren
fluorometric (Luminex xMAP)
Versandbedingung
wet ice
Allgemeine Beschreibung
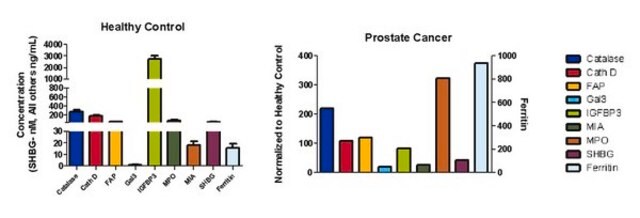
Metastasis is a complex process in which tumor cells leave the primary tumor site and migrate to other parts of the body via blood and lymph vessels or by tumor invasion of surrounding tissues and organs. Breaking away from the primary tumor, cancer cells attach to and degrade extracellular matrix proteins that separate tumor from surrounding tissues to escape into the stroma. Where metastases form often depends on the primary tumor. Bone metastases, for example, tend to arise from breast, prostate and kidney tumors. This organ/tissue specificity seems to involve chemical signals from both chemokines and growth factors.
Panel Type: Circulating Cancer
Anwendung
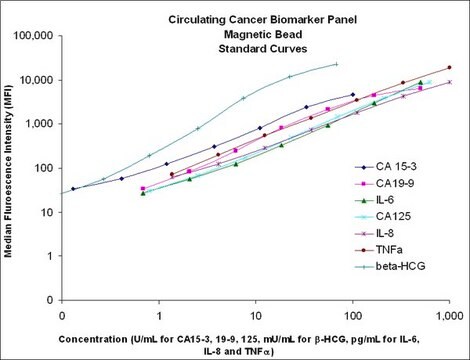
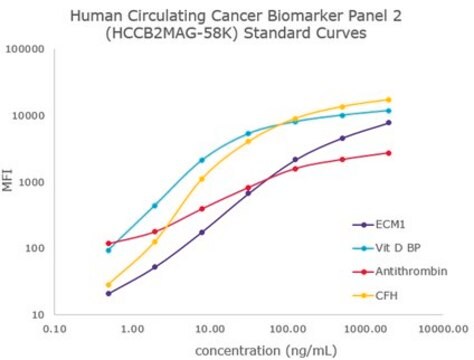
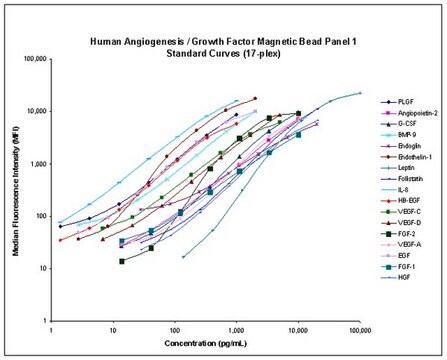
- Analytes: DKK-1, GDF-15, Neuron-specific Enolase (NSE), Osteonectin (SPARC), Osteoprotegerin (OPG), Periostin, TRAP5, TWEAK, YKL40 (CHI3L1)
- Recommended Sample Type: Human serum, plasma, and tissue/cell culture supernatants or lysates
- Recommended Sample Dilution: 25 μL of 1:10 diluted serum or plasma; tissue/cell culture supernatants or lysates diluted may be diluted as needed in appropriate control medium
- Assay Run Time: Overnight (16-18 hours) at 2-8°C
- Research Category: Cancer
Leistungsmerkmale und Vorteile
Sonstige Hinweise
Rechtliche Hinweise
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Sens. 1 - STOT RE 2
Zielorgane
Respiratory Tract
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.