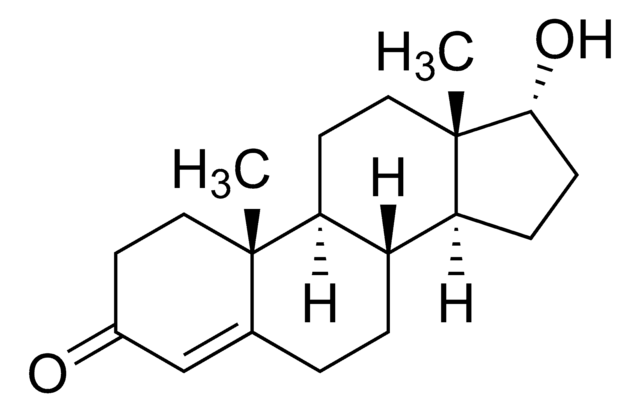
H-059
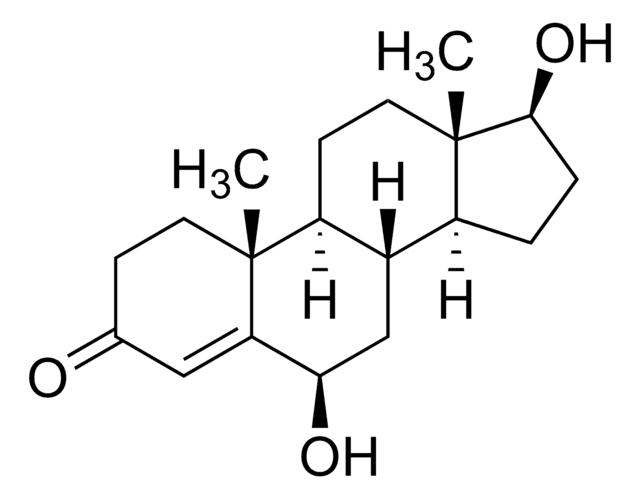
6β-Hydroxytestosterone solution
100 μg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Empfohlene Produkte
Qualität
certified reference material
Qualitätsniveau
Form
liquid
Leistungsmerkmale
Snap-N-Spike®/Snap-N-Shoot®
Verpackung
ampule of 1 mL
Hersteller/Markenname
Cerilliant®
Konzentration
100 μg/mL in methanol
Methode(n)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Anwendung(en)
clinical testing
Format
single component solution
Lagertemp.
−20°C
SMILES String
C[C@]12CC[C@H]3[C@@H](C[C@@H](O)C4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2O
InChI
1S/C19H28O3/c1-18-7-5-11(20)9-15(18)16(21)10-12-13-3-4-17(22)19(13,2)8-6-14(12)18/h9,12-14,16-17,21-22H,3-8,10H2,1-2H3/t12-,13-,14-,16+,17-,18+,19-/m0/s1
InChIKey
XSEGWEUVSZRCBC-ZVBLRVHNSA-N
Allgemeine Beschreibung
Anwendung
- Investigating enzyme inhibition with 6β-Hydroxytestosterone: The study on liver microsomal bioreactors for rapid drug metabolism and inhibition assays employed 6β-Hydroxytestosterone to understand its effects on CYP450 enzyme activities, highlighting its utility in pharmacokinetic studies and enzyme behavior under varying physiological conditions (Walgama et al., 2015).
- 6β-Hydroxytestosterone as a biochemical assay reagent: A sensitive and specific LC-MS/MS cocktail assay developed for CYP450 enzymes employed 6β-Hydroxytestosterone as a reference compound, demonstrating its applicability in biochemical assays and its role in enhancing the detection of enzymatic activity and metabolic pathways (Nguyen et al., 2020).
Rechtliche Hinweise
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Zielorgane
Eyes
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 2
Flammpunkt (°F)
49.5 °F - closed cup
Flammpunkt (°C)
9.7 °C - closed cup
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Separation of Testosterone solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; 17α-Methyltestosterone solution, 1.0 mg/mL in 1,2-dimethoxyethane, ampule of 1 mL, certified reference material; 5α-Dihydrotestosterone (DHT) solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; 6β-Hydroxytestosterone solution, 100 μg/mL in methanol, ampule of 1 mL, certified reference material
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.