810227P
Avanti
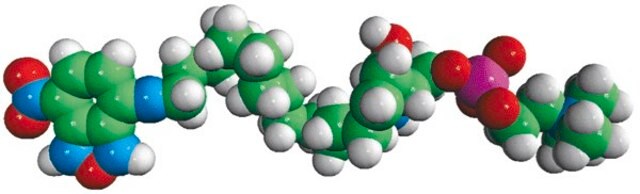
C12-NBD Lactosyl Ceramide
Avanti Research™ - A Croda Brand 810227P, powder
Synonym(e):
N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-D-lactosyl-β1-1′-sphingosine
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C48H81N5O16
CAS-Nummer:
Molekulargewicht:
984.18
UNSPSC-Code:
12352211
NACRES:
NA.25
Empfohlene Produkte
Assay
>99% (TLC)
Form
powder
Verpackung
pkg of 1 × 50 μg (810227P-50ug)
Hersteller/Markenname
Avanti Research™ - A Croda Brand 810227P
Versandbedingung
dry ice
Lagertemp.
−20°C
Allgemeine Beschreibung
C12-NBD Lactosyl ceramide is a fluorescent analog of biologically available compound lactosyl ceramide. Lactosyl ceramideis generally present on neutrophils and macrophages.
Biochem./physiol. Wirkung
Lactosyl ceramide is the major precursor for synthesis of various glycosphingolipids like oligoglycosylceramides and gangliosides. It is an important signaling molecule that is involved in adhesion, migration, cell proliferation and angiogenesis. Lactosyl ceramide is vital for osteoclastogenesis mediated by macrophage-colony stimulating factor.
Verpackung
5 mL Amber Glass Screw Cap Vial (810227P-50ug)
Rechtliche Hinweise
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Lot/Batch Number
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
T Iwamoto et al.
The Journal of biological chemistry, 276(49), 46031-46038 (2001-10-11)
Glycosphingolipids and their metabolites play important roles in a variety of biological processes. Several signal molecules are localized in a glycolipid-enriched microdomain on the cell surface, and their signals are regulated by the glycolipid composition. However, the function of glycolipids
W I Weis et al.
Annual review of biochemistry, 65, 441-473 (1996-01-01)
Lectins are responsible for cell surface sugar recognition in bacteria, animals, and plants. Examples include bacterial toxins; animal receptors that mediate cell-cell interactions, uptake of glycoconjugates, and pathogen neutralization; and plant toxins and mitogens. The structural basis for selective sugar
S Hakomori et al.
Journal of biochemistry, 118(6), 1091-1103 (1995-12-01)
Glycosphingolipids (GSLs), cell type-specific markers which change dramatically during ontogenesis and oncogenesis, have been implicated as playing major roles in cellular interactions and control of cell proliferation in multicellular organisms. These functional roles have been partially clarified through two types
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.