Wichtige Dokumente
W318101
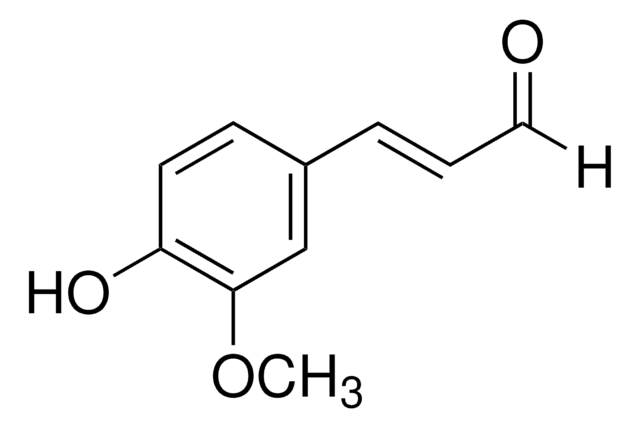
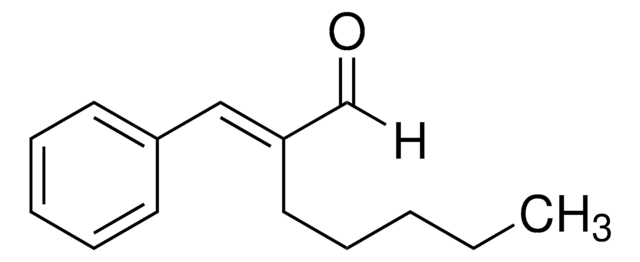
2-Methoxycinnamaldehyde
natural, 98%, FG
Synonym(e):
o-Methoxycinnamaldehyde
About This Item
Empfohlene Produkte
Qualität
FG
Fragrance grade
Halal
Kosher
natural
Qualitätsniveau
Agentur
follows IFRA guidelines
Einhaltung gesetzlicher Vorschriften
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Assay
98%
Grünere Alternativprodukt-Eigenschaften
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
bp
160-161 °C/12 mmHg (lit.)
mp (Schmelzpunkt)
44.0-49.0 °C (lit.)
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Allergener Duftstoff
no known allergens
Grünere Alternativprodukt-Kategorie
Organoleptisch
cinnamon; woody; spicy; sweet
SMILES String
[H]C(=O)C=Cc1ccccc1OC
InChI
1S/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3/b6-4+
InChIKey
KKVZAVRSVHUSPL-GQCTYLIASA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- Network pharmacology combined with molecular docking and experimental validation to explore the potential mechanism of Cinnamomi ramulus against ankylosing spondylitis.: This study investigates the anti-inflammatory potential of 2-Methoxycinnamaldehyde in Cinnamomi ramulus. Its application extends to novel therapeutic approaches for treating ankylosing spondylitis, demonstrating significant implications for medicinal chemistry and pharmacology (Wei et al., 2023).
- Ramulus Cinnamomi essential oil exerts an anti-inflammatory effect on RAW264.7 cells through N-acylethanolamine acid amidase inhibition.: The study elaborates on the anti-inflammatory activities of 2-Methoxycinnamaldehyde, offering a biochemical pathway that could be exploited in anti-inflammatory drug design (Jia et al., 2023).
- Metabolomics-Driven Exploration of the Antibacterial Activity and Mechanism of 2-Methoxycinnamaldehyde.: This article offers insights into the antibacterial properties of 2-Methoxycinnamaldehyde, using metabolomics to explore its mechanism of action, significant for developments in antimicrobial treatments (Qian et al., 2022).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
235.4 °F
Flammpunkt (°C)
113 °C
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W318101-100G-K | 4061837528002 |
| W318101-1KG-K | 4061834365853 |
| W318101-10KG-K | 4061833905906 |
| W318101-25KG-K | 4061833905913 |
| W318101-SAMPLE-K | 4061834356080 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.