Wichtige Dokumente
H17082
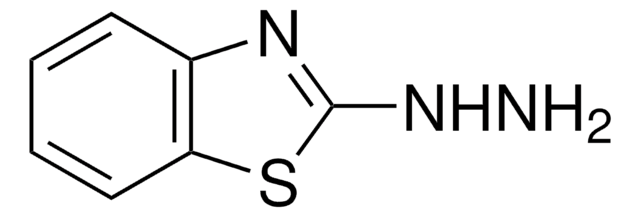
2-Hydrazinopyridin
97%
Synonym(e):
2-Pyridylhydrazin
About This Item
Empfohlene Produkte
Assay
97%
bp
90-92 °C/1 mmHg (lit.)
mp (Schmelzpunkt)
41-44 °C (lit.)
Lagertemp.
2-8°C
SMILES String
NNc1ccccn1
InChI
1S/C5H7N3/c6-8-5-3-1-2-4-7-5/h1-4H,6H2,(H,7,8)
InChIKey
NWELCUKYUCBVKK-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Enhanced Metal Removal: Utilizing Schiff base functionalized dialdehyde starch, derived from 2-hydrazinopyridine, demonstrated significant potential in enhancing the removal of Cu(II) from solutions. The study included preparation methodologies, performance evaluations, and DFT calculations, showcasing its efficacy in water treatment technologies (Liang et al., 2024).
- Dual Sensing Probe Development: A novel dicyanisophorone-based probe, incorporating 2-hydrazinopyridine, was developed for the dual sensing of Zn(2+) and Cd(2+) via near-infrared fluorescence. This advancement aids in the detection and analysis of heavy metals in various environmental and biological samples (Yan et al., 2023).
- Active Site Analysis in Lysyl Oxidase: The study provided insights into the spatial arrangement of active site components in Lysyl Oxidase-like 2, including the role of 2-hydrazinopyridine, which is critical for understanding the enzyme′s mechanism and potential therapeutic applications (Meier et al., 2022).
- Structural Analysis of Lysyl Oxidase: Research focused on the predicted 3D structure of the amine oxidase domain of Lysyl Oxidase-Like 2, exploring the interaction dynamics facilitated by 2-hydrazinopyridine. This contributes significantly to the field of molecular biology and enzyme function analysis (Meier et al., 2022).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
230.0 °F - closed cup
Flammpunkt (°C)
110 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.