901741
Bis(triphenyl-λ5-phosphanylidene)ammonium hydrogendifluoride
Synonym(e):
Bifluoride salt SuFEx catalyst, PNP+[FHF]-, Triphenyl((triphenyl-phosphaneylidene)amino)phosphonium fluoride hydrofluoride, [Ph3P=N-PPh3]+[HF2]-
About This Item
Empfohlene Produkte
Assay
≥95%
Form
solid
Eignung der Reaktion
reagent type: catalyst
Grünere Alternativprodukt-Eigenschaften
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Grünere Alternativprodukt-Kategorie
Lagertemp.
2-8°C
Allgemeine Beschreibung
Anwendung
Ähnliches Produkt
Lagerklassenschlüssel
13 - Non Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Verwandter Inhalt
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.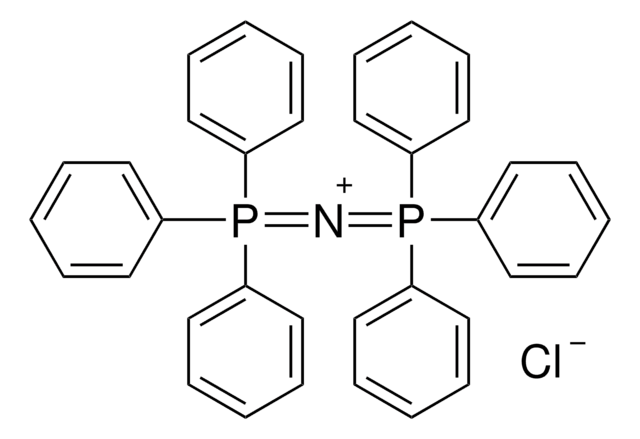
![Tetrakis[tris(dimethylamino)phosphoranylidenamino]phosphoniumchlorid 95%](/deepweb/assets/sigmaaldrich/product/structures/160/963/9dd6d457-17b2-44dc-8ea2-d3c0475b3664/640/9dd6d457-17b2-44dc-8ea2-d3c0475b3664.png)
![2-[(Trimethylsilyl)ethinyl]anilin 97%](/deepweb/assets/sigmaaldrich/product/structures/194/066/182b08e4-35d7-4b7b-8958-c917f64391fc/640/182b08e4-35d7-4b7b-8958-c917f64391fc.png)