901635
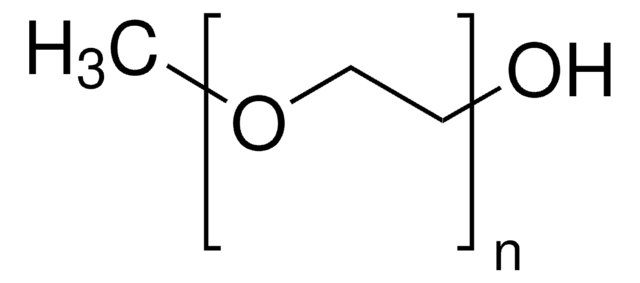
Poly(ethylene glycol) bis(2-pyridyl KAT)
PEG average Mn 10,000
Synonym(e):
KAT PEG, bis KAT PEG, di KAT PEG
About This Item
Empfohlene Produkte
Form
powder or solid
Mol-Gew.
PEG average Mn 10,000
PEG ~10,000 Da
Farbe
off-white to pale yellow
Lagertemp.
2-8°C
Allgemeine Beschreibung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Verwandter Inhalt
The Bode Group aims to develop new reactions and reagents for the synthesis of complex molecules. The Bode Group has developed N-mesityl-substituted NHCs as organocatalysts for the catalytic generation of reactive species including activated carboxylates, homoenolates, and enolates. These novel catalysts and reactions have made possible a new generation of highly enantioselective annulations from simple starting materials under mild reaction conditions, usually at room temperature and without added reagents. Furthering the goal of designing new reagents to enable the assembly of complex molecules, the Bode group has developed SnAP reagents for the facile, one-pot conversion of aldehydes into N-unprotected, saturated N-heterocycles, including bicyclic and spirocyclic structures. These easy to handle reagents provide a simple and robust alternative to the challenging and restrictive cross-coupling methods for the functionalization of saturated N-heterocycles.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.