Wichtige Dokumente
569593
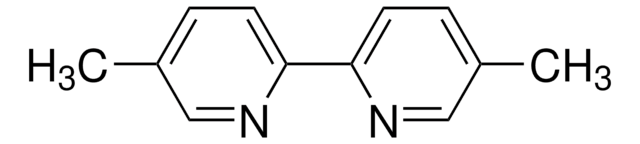
4,4′-Dimethyl-2,2′-dipyridyl
99.5%, purified by sublimation
Synonym(e):
2,2′-Bi-(γ-picolin), 4,4′-Dimethyl-2,2′-bipyridin
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
99.5%
Form
solid
Aufgereinigt durch
sublimation
mp (Schmelzpunkt)
169-174 °C (lit.)
SMILES String
Cc1ccnc(c1)-c2cc(C)ccn2
InChI
1S/C12H12N2/c1-9-3-5-13-11(7-9)12-8-10(2)4-6-14-12/h3-8H,1-2H3
InChIKey
NBPGPQJFYXNFKN-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- carbonato-bridged triangular trinuclear compounds and tetranuclear hydroxo-bridged compounds
- dmbipy-copper bridged complexes, which show variation in crystal geometries, thermal and magnetic properties
Ähnliches Produkt
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
Tools and techniques for performing atom transfer radical polymerization (ATRP) with benefits and limitations.
Tools for Performing ATRP
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Protokolle
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Sigma-Aldrich presents an article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.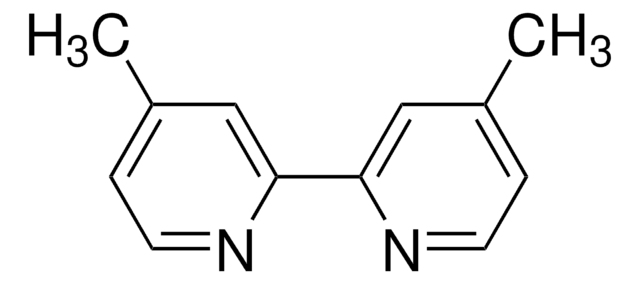




![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)