550426
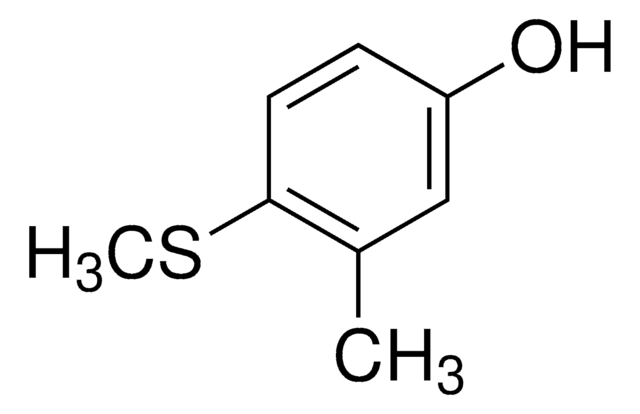
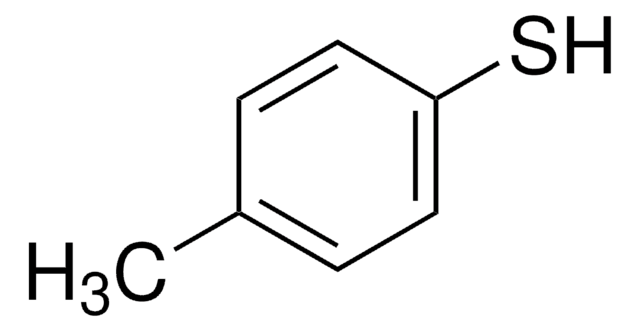
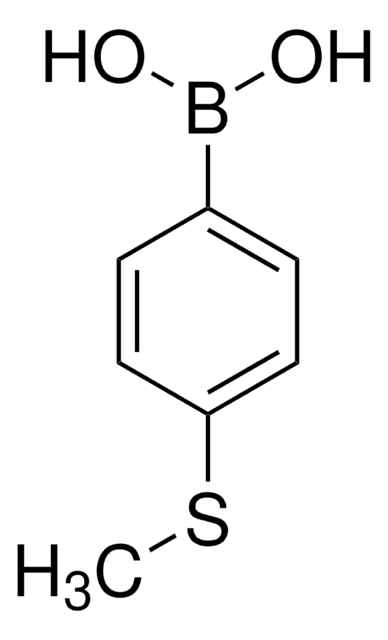
4-(Methylmercapto)phenol
98%
Synonym(e):
4-Hydroxythioanisol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
CH3SC6H4OH
CAS-Nummer:
Molekulargewicht:
140.20
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
bp
153-156 °C/20 mmHg (lit.)
mp (Schmelzpunkt)
84-86 (lit.)
SMILES String
CSc1ccc(O)cc1
InChI
1S/C7H8OS/c1-9-7-4-2-6(8)3-5-7/h2-5,8H,1H3
InChIKey
QASBCTGZKABPKX-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
4-(Methylmercapto)phenol (4MP) mediates the removal of benzyloxycarbonyl and O-benzyl protecting groups by accepting the benzyl groups during the acidolytic cleavage with trifluoroacetic acid. The presence of hydroxyl group in the para position enhances the rate of hydrodesulfurization (HDS) of 4MP.
Anwendung
4-(Methylmercapto)phenol [4-(Methylthio)phenol] may be used in the preparation of phosphoramidodithioate intermediates for the synthesis of sulprofos amidate.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Lianming Wu et al.
Journal of mass spectrometry : JMS, 44(9), 1389-1394 (2009-08-22)
A novel ion/molecule reaction was observed to occur under electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and atmospheric pressure photo ionization (APPI) conditions, leading to dimerization of ionized 4-(methyl mercapto)-phenol followed by fast H(*) loss. The reaction is particularly
Acceptors in the removal of protecting groups.
Bodanszky M and Bodanszky A.
International Journal of Peptide and Protein Research, 23(3), 287-291 (1984)
Hydrotreating of compounds containing both oxygen and sulfur: effect of para-hydroxyl substituent on the reactions of mercapto and methylmercapto groups.
Viljava TR and Krause AOI.
Applied Catalysis A: General, 145(1), 237-251 (1996)
Resolution and stereoselective action of sulprofos and related S-propyl phosphorothiolates.
Hirashima A, et al.
Journal of Agricultural and Food Chemistry, 32(6), 1302-1307 (1984)
Hua Zhang et al.
Molecules (Basel, Switzerland), 15(1), 83-92 (2010-01-30)
A highly efficient transition-metal-free catalytic system Br2/NaNO2/H2O has been developed for a robust and economic acid-free aerobic oxidation of sulfides. It is noteworthy that the sulfide function reacts under mild conditions without over-oxidation to sulfone. The role of NaNO2as an
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.