Alle Fotos(3)
Wichtige Dokumente
431966
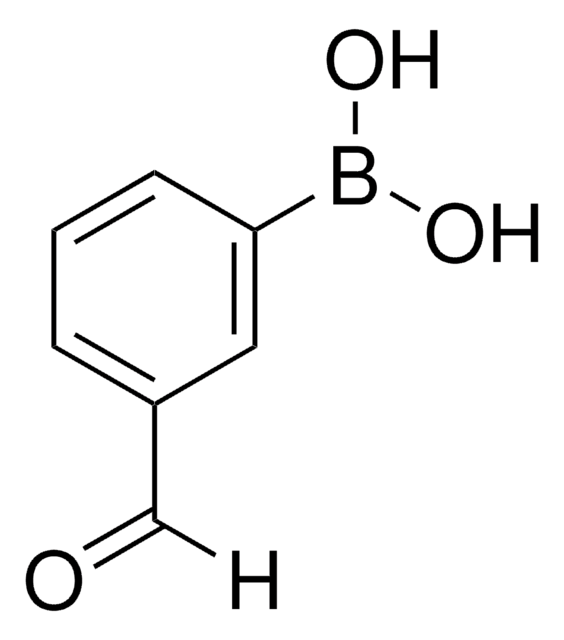
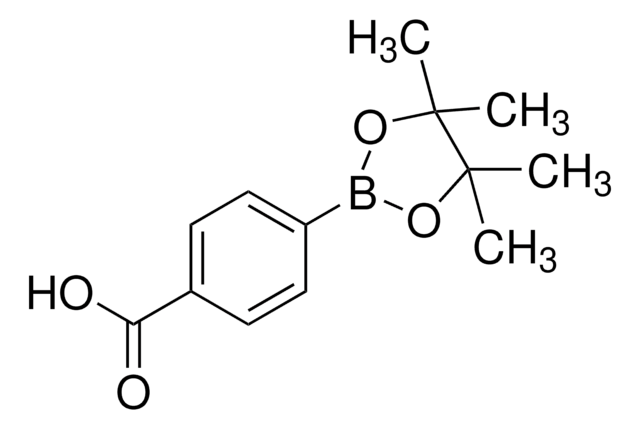
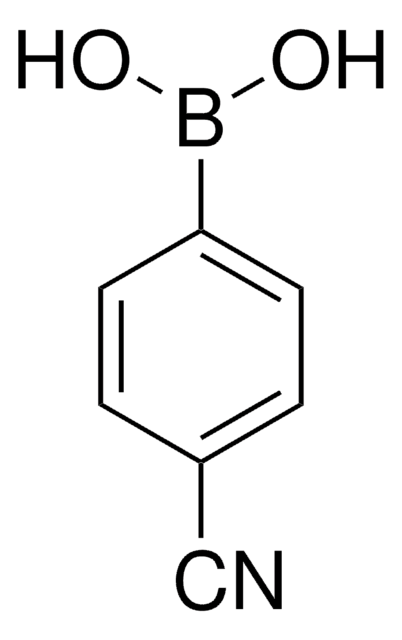
4-Formylphenylborsäure
≥95.0%
Synonym(e):
4-(Dihydroxyboryl)-benzaldehyd, 4-Borono-benzaldehyd
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Lineare Formel:
HCOC6H4B(OH)2
CAS-Nummer:
Molekulargewicht:
149.94
Beilstein:
3030770
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥95.0%
mp (Schmelzpunkt)
237-242 °C (lit.)
Funktionelle Gruppe
aldehyde
SMILES String
OB(O)c1ccc(C=O)cc1
InChI
1S/C7H7BO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5,10-11H
InChIKey
VXWBQOJISHAKKM-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
4-Formylphenylboronic acid is a substrate for Suzuki cross-coupling reactions and it can be used as a reagent for:
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Triethylamine-catalyzed three-component Hantzsch condensations.
- Copper-catalyzed nitrations.
- Oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta.
- Palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides.
- Palladium-catalyzed aerobic oxidative cross-coupling reactions.
- The synthesis of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells.
- The synthesis of a novel protein synthesis inhibitor active against Gram-positive bacteria.
- The Suzuki aryl-aryl coupling of the upper rim of hexahomotrioxacalix[3]arene.
- A rhodium-catalyzed cyclization, converting 1,5-enynes to cyclopentenes and spiro-cyclopentenes.
Sonstige Hinweise
Contains varying amounts of anhydride
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Skin Sens. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Synthesis of Some New 1,4-Dihydropyridine Derivatives through a Facile One-pot Hantzsch Condensation Catalyzed by Triethylamine
Chin. J. Chem., 30, 733-737 (2012)
Kunpeng Guo et al.
Organic letters, 14(9), 2214-2217 (2012-04-14)
This work identifies the dithiafulvenyl unit as an excellent electron donor for constructing D-π-A-type metal-free organic sensitizers of dye-sensitized solar cells (DSCs). Synthesized and tested are three sensitizers all with this donor and a cyanoacrylic acid acceptor but differing in
Tetrahedron, 62, 10321-10321 (2006)
Nora R Eibergen et al.
Chembiochem : a European journal of chemical biology, 13(4), 574-583 (2012-03-01)
In an effort to identify novel antibacterial chemotypes, we performed a whole-cell screen for inhibitors of Staphylococcus aureus growth and pursued those compounds with previously uncharacterized antibacterial activity. This process resulted in the identification of a benzothiazolium salt, ABTZ-1, that
Qi Huang et al.
ACS applied materials & interfaces, 11(17), 15861-15868 (2019-03-28)
Conjugated microporous polymers (CMPs) with high surface areas, tunable building blocks, and fully conjugated structures have found important applications in optoelectronics. Here, we report a new series of CMPs with tunable band gaps by introducing thiazolo[5,4- d] thiazole as the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 431966-1G | 4061832108520 |
| 431966-25G | 4061832108537 |
| 431966-5G | 4061832108544 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.