Alle Fotos(1)
Wichtige Dokumente
422916
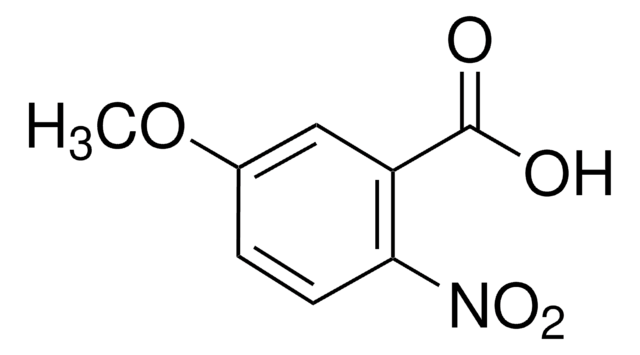
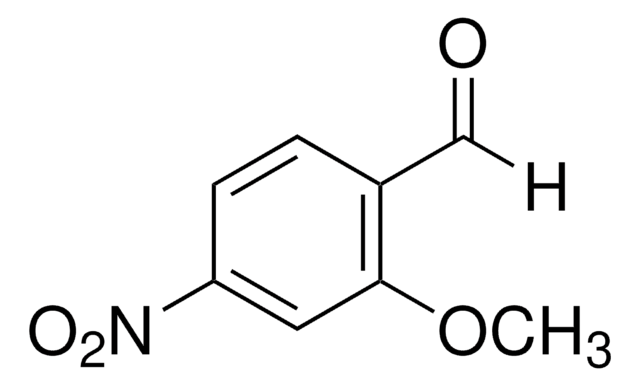
2-Methoxy-4-nitrobenzoesäure
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
CH3OC6H3(NO2)CO2H
CAS-Nummer:
Molekulargewicht:
197.14
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
mp (Schmelzpunkt)
146-148 °C (lit.)
Funktionelle Gruppe
carboxylic acid
nitro
SMILES String
COc1cc(ccc1C(O)=O)[N+]([O-])=O
InChI
1S/C8H7NO5/c1-14-7-4-5(9(12)13)2-3-6(7)8(10)11/h2-4H,1H3,(H,10,11)
InChIKey
KPJXEWJRJKEOCD-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
2-Methoxy-4-nitrobenzoic acid is an alkoxybenzoic acid derivative. It has been reported to be synthesized by reacting 2-hydroxy-4-nitrobenzoic acid with methyl iodide and characterized by 1H and 13C-NMR spectra.
Anwendung
2-Methoxy-4-nitrobenzoic acid may be used as a starting material in the following syntheses:
- 2-Methoxy-4-nitrobenzamide, a nitroamide derivative.
- 4-Amino-2-methoxybenzamide, an aminobenzamide derivative.
- N,2-Dimethoxy-N-methyl-4-nitrobenzamide, a Weinreb amide derivative.
- 1-(2-Methoxy-4-nitrophenyl)-5-methylhex-2-yn-1-one, a ynone derivative.
- 2,5-Constrained piperidine derivative, a potential CCR3 (Chemokine (C-C Motif) Receptor 3) antagonist.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Marc J Adler et al.
The Journal of organic chemistry, 76(17), 7040-7047 (2011-07-12)
The design and synthesis of small molecule α-helix mimetics has been a productive field over the past decade. These compounds have performed well in a variety of biological systems as functional disruptors of α-helix-mediated protein-protein interactions. In our studies we
Leyi Gong et al.
Bioorganic & medicinal chemistry letters, 13(20), 3597-3600 (2003-09-25)
As part of our investigation into the development of potent CCR3 antagonists, a series of piperidine analogues was designed and prepared. Exploration of the piperidine core examined both the basicity and the location of a nitrogen, as well as conformational
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.