Wichtige Dokumente
392251
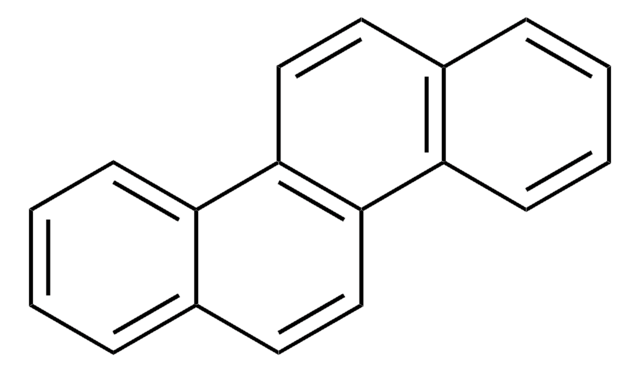
Benzo[k]fluoranthen
for fluorescence, ≥99%
Synonym(e):
11,12-Benzofluoranthene, 2,3,1′,8′-Binaphthylene, 8,9-Benzfluoranthene
About This Item
Empfohlene Produkte
Qualität
for fluorescence
Qualitätsniveau
Assay
≥99%
mp (Schmelzpunkt)
215-217 °C (lit.)
Löslichkeit
95% ethanol: <1 mg/mL at 20 °C
DMSO: <1 mg/mL at 20 °C
H2O: <1 mg/mL at 20 °C
acetone: 1-10 mg/mL at 20 °C
methanol: <1 mg/mL at 20 °C
toluene: 5-10 mg/mL at 20 °C
Eignung
suitable for fluorescence
SMILES String
c1ccc2cc-3c(cc2c1)-c4cccc5cccc-3c45
InChI
1S/C20H12/c1-2-6-15-12-19-17-10-4-8-13-7-3-9-16(20(13)17)18(19)11-14(15)5-1/h1-12H
InChIKey
HAXBIWFMXWRORI-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Enhanced degradation of carcinogenic PAHs benzo (a) pyrene and benzo (k) fluoranthene by a microbial consortia: Bioremediation of high molecular weight PAHs with a combination of microorganisms (S Guntupalli, V Thunuguntla, 2016).
- Biotransformation of the high‐molecular weight polycyclic aromatic hydrocarbon (PAH) benzofluoranthene by Sphingobium sp. strain KK22 and identification of metabolites: Biotransformation and identification of products from benzo[k]fluoranthene (AH Maeda, S Nishi, Y Hatada, Y Ozeki, 2014).
- Investigation of the electrochemical properties of benzofluorenthene using a glassy carbon electrode and development of a square-wave voltammetric method for detection: Electrochemical behavior of benzo[k]fluorenthene and development of detection method (A Altun, Y Yardim, A Levent, 2023).
Verpackung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Persönliche Schutzausrüstung
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Zulassungslistungen
Zulassungslistungen werden hauptsächlich für chemische Produkte erstellt. Für nicht-chemische Produkte können hier nur begrenzte Angaben gemacht werden. Kein Eintrag bedeutet, dass keine der Komponenten gelistet ist. Es liegt in der Verantwortung des Benutzers, die sichere und legale Verwendung des Produkts zu gewährleisten.
EU REACH SVHC Candidate List
EU REACH Annex XVII (Restriction List)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 392251-100MG | 4061833279854 |
| 392251-25G | |
| 392251-25MG |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.![Benzo[b]fluoranthen 98%](/deepweb/assets/sigmaaldrich/product/structures/175/744/6fa5fca2-b6ec-47b6-ab7a-fe895843f226/640/6fa5fca2-b6ec-47b6-ab7a-fe895843f226.png)
![Benzo[ghi]perylen 98%](/deepweb/assets/sigmaaldrich/product/structures/154/740/c50ff1be-dfb4-4159-a98c-9cecf9206ad3/640/c50ff1be-dfb4-4159-a98c-9cecf9206ad3.png)
![Indeno[1,2,3-cd]pyren analytical standard](/deepweb/assets/sigmaaldrich/product/structures/231/153/b0b230c2-efa0-4f43-a261-66b931ead3d2/640/b0b230c2-efa0-4f43-a261-66b931ead3d2.png)
![Dibenz[a,h]anthracen certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland](/deepweb/assets/sigmaaldrich/product/structures/358/871/0a80ecfc-d123-44ca-90a6-22248b43aba9/640/0a80ecfc-d123-44ca-90a6-22248b43aba9.png)
![Benzo[a]pyren ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)

![Benzo[k]fluoranthen certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland](/deepweb/assets/sigmaaldrich/product/structures/277/320/3e615f9f-3887-40f6-b176-bc1eb9b4832c/640/3e615f9f-3887-40f6-b176-bc1eb9b4832c.png)


![Benzo[b]fluoranthen-d12 98 atom % D](/deepweb/assets/sigmaaldrich/product/structures/111/602/8ca7df58-767a-4f37-a19b-2bebdc865669/640/8ca7df58-767a-4f37-a19b-2bebdc865669.png)