Alle Fotos(1)
Wichtige Dokumente
392189
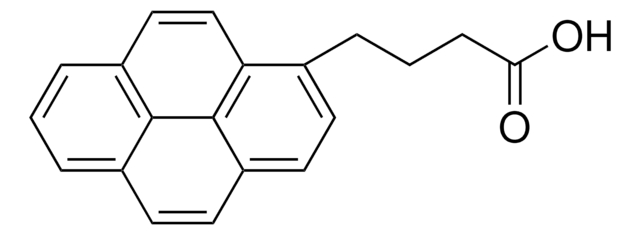
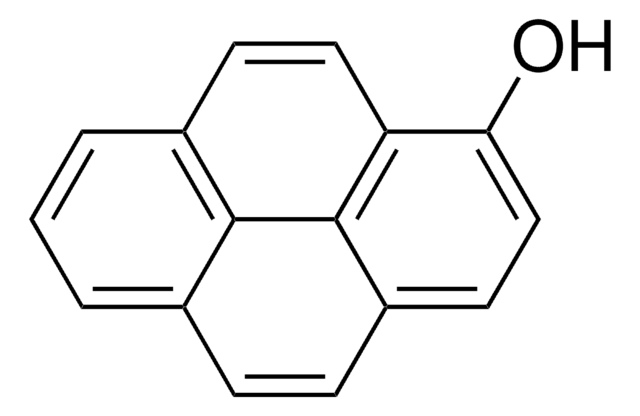
1-Pyrenessigsäure
97%
Synonym(e):
(1-Pyrenyl)acetic acid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C18H12O2
CAS-Nummer:
Molekulargewicht:
260.29
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
Form
solid
mp (Schmelzpunkt)
210-212 °C (dec.) (lit.)
Funktionelle Gruppe
carboxylic acid
SMILES String
OC(=O)Cc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O2/c19-16(20)10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)18(13)17(11)12/h1-9H,10H2,(H,19,20)
InChIKey
SDJCLYBBPUHKCD-UHFFFAOYSA-N
Allgemeine Beschreibung
1-Pyreneacetic acid is a negatively charged pyrene derivative. It has been proposed as titrating reagent for the standardization titration of Grignard reagents and n-butyl lithium (n-BuLi).
Anwendung
1-Pyreneacetic acid is suitable for use in the following studies:
- Synthesis of N-(2-(methylthio)ethyl)-2-(pyren-1-yl)acetamide, a pyrene amide based Pd2+ probe.
- Synthesis of pyrene-modified β-cyclodextrin.
- To functionalize single walled carbon nanotube field effect transistors (CNT FETs).
- As an agent for characterizing grafting degrees and reactivity of the ester functionalized polypropylenes.
- Synthesis sawhorse-type diruthenium tetracarbonyl complexes.
- Synthesis of (±)-2-(1-pyrenyl)propionic acid, a chiral carboxylic acid.
- Reversible noncovalent functionalization of single walled carbon nanotubes (SWNTs).
- Preparation of peptide nucleic acid (PNA) probes.
- As an internal reference compound in the assessment of solid phase reaction by HPLC-UV.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Jan Spengler et al.
ACS combinatorial science, 15(5), 229-234 (2013-03-26)
Here we evaluated the use of internal reference compounds for the rapid assessment of reactions performed in solid-phase. An internal reference compound (commercially available) was bound to the resin, together with the substrate, and cleaved with the products after completion
Sawhorse-type diruthenium tetracarbonyl complexes derived from pyrenyl-carboxylic acids.
Johnpeter JP and Therrien B.
Inorgorganica Chimica Acta, 405, 437-443 (2013)
Gabriela Ramos Chagas et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(23), 3429-3436 (2017-09-01)
A smart stimuli-responsive surface was fabricated by the electro-copolymerization of pyrene monomers followed by base and acid treatment. Copolymers of pyrenes bearing fluorinated chains (Py-nF
Alex Manicardi et al.
Beilstein journal of organic chemistry, 10, 1495-1503 (2014-08-28)
Pyrene derivatives can be incorporated into nucleic acid analogs in order to obtain switchable probes or supramolecular architectures. In this paper, peptide nucleic acids (PNAs) containing 1 to 3 1-pyreneacetic acid units (PNA1-6) with a sequence with prevalence of pyrimidine
Murphy PJ.
Organophosphorus Reagents: A Practical Approach in Chemistry, 8-8 (2004)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.