Alle Fotos(2)
Wichtige Dokumente
383449
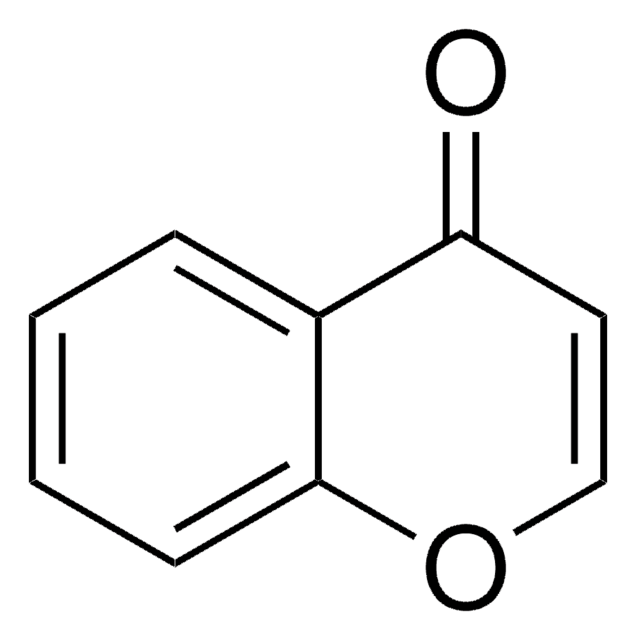
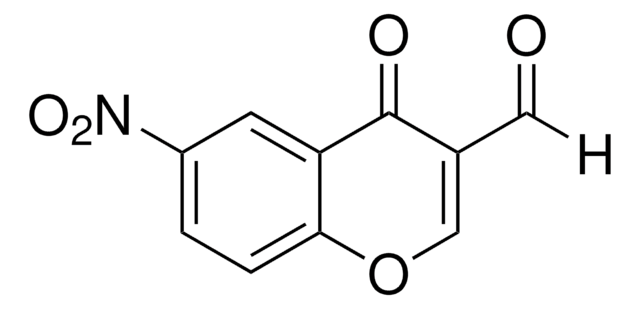
3-Formylchromon
97%
Synonym(e):
4-Oxo-4H-1-benzopyran-3-carboxaldehyd
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C10H6O3
CAS-Nummer:
Molekulargewicht:
174.15
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
Form
solid
mp (Schmelzpunkt)
151-153 °C (lit.)
Funktionelle Gruppe
aldehyde
ketone
SMILES String
O=CC1=COc2ccccc2C1=O
InChI
1S/C10H6O3/c11-5-7-6-13-9-4-2-1-3-8(9)10(7)12/h1-6H
InChIKey
FSMYWBQIMDSGQP-UHFFFAOYSA-N
Angaben zum Gen
human ... PTPN1(5770)
Allgemeine Beschreibung
Electrospray ionization mass spectrometry (ESI-MS) of protonated 3-formylchromone (3-FC) shows loss of H2 as a major fragmentation route to yield a ketene cation, which on reaction with water forms a protonated carboxylic acid. The invivo salubrious effects of 3-FC against nitrosodiethylamine (NDEA) mediated early hepatocellular carcinogenesis has been investigated. Synthesis and characterization of 3-FC and its derivatives has been reported.
Anwendung
3-Formylchromone may be used in the following studies:
- Preparation of library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones, by three-component domino reactions with (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones and anilines under catalyst-free conditions.
- Facile and ecofriendly synthesis of new chromonyl chalcones.
- Synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Koichi Takao et al.
Bioorganic chemistry, 83, 432-437 (2018-11-15)
A series of eighteen pyrano[4,3-b][1]benzopyranone derivatives (1a-9b) were synthesized, and structure-activity relationships of their monoamine oxidase (MAO) A and B, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) inhibitory activities were evaluated. Most of the synthesized compounds exhibited weak inhibitory activity toward MAO-A
Pedatsur Neta et al.
Rapid communications in mass spectrometry : RCM, 28(17), 1871-1882 (2014-08-05)
Electrospray ionization mass spectrometry (ESI-MS) of many protonated aldehydes shows loss of CO as a major fragmentation pathway. However, we find that certain aldehydes undergo loss of H2 followed by reaction with water in the collision cell. This complicates interpretation
Andrey S Plaskon et al.
The Journal of organic chemistry, 73(15), 6010-6013 (2008-07-03)
A facile and versatile procedure for the synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones was elaborated on the basis of TMSCl-mediated recyclization of 3-formylchromone with various anilines. Limitations and scope of this methodology were established, and a possible mechanism for the heterocyclizations
Pitchaimani Prasanna et al.
Beilstein journal of organic chemistry, 10, 459-465 (2014-03-13)
The three-component domino reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, 3-formylchromone and anilines under catalyst-free conditions afforded a library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones in good to excellent yields and in a diastereoselective transformation. This transformation generates one C-C and one C-N bond and presumably proceeds
Zeba N Siddiqui et al.
Journal of enzyme inhibition and medicinal chemistry, 27(1), 84-91 (2011-05-27)
A facile and ecofriendly synthesis of new chromonyl chalcones 3a-b from 3-formylchromone 1 and active methyl compounds 2a-b is reported under thermal solvent-free heating condition in good yields. The chromonyl chalcones 3a-b were used as intermediates under green condition for
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.