Alle Fotos(1)
Wichtige Dokumente
302694
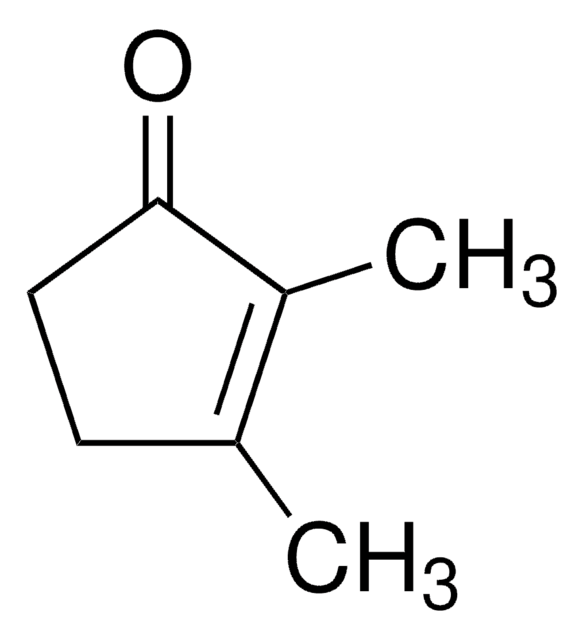
3-Acetyl-2,5-dimethylfuran
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C8H10O2
CAS-Nummer:
Molekulargewicht:
138.16
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Brechungsindex
n20/D 1.485 (lit.)
bp
62 °C/0.25 mmHg (lit.)
Dichte
1.038 g/mL at 25 °C (lit.)
Funktionelle Gruppe
ketone
SMILES String
CC(=O)c1cc(C)oc1C
InChI
1S/C8H10O2/c1-5-4-8(6(2)9)7(3)10-5/h4H,1-3H3
InChIKey
KBSVBCHYXYXDAG-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
3-Acetyl-2,5-dimethylfuran is a flavouring agent and its specifications were revised.
Anwendung
3-Acetyl-2,5-dimethylfuran was used in synthesis of:
- furylfulgide, 2-[1-(2, 5-dimethyl-3-furyl) ethylidene]-3-isopropylidene succinic anhydride
- chalcones
- 1-(2′,5′-dimethyl-3′-furyl)-3-(aryl)-2-propen-1-one
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
174.2 °F - closed cup
Flammpunkt (°C)
79 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Photochromism of a furylfulgide, 2-[1-(2, 5-dimethyl-3-furyl) ethylidene]-3-isopropylidene succinic anhydride in solvents and polymer films
Yokoyama Y, et al.
Bulletin of the Chemical Society of Japan, 63(6), 1607-1610 (1990)
SYNTHESIS AND STUDY OF ANTI-INFLAMMATORY AND ANTIMICROBIAL ACTIVITY OF SOME NEW 1, 3, 5-TRISUBSTITUTED PYRAZOLINES.
Sridhar S, et al.
International Journal of Chemical Sciences, 8(4), 2697-2707 (2010)
J Bend et al.
World Health Organization technical report series, (947)(947), 1-225 (2008-06-17)
This report represents the conclusions of a Joint FAO/WHO Expert Committee convened to evaluate the safety of various food additives, including flavouring agents, with a view to recommending acceptable daily intakes (ADIs) and to preparing specifications for identity and purity.
Maura Koehle et al.
ChemSusChem, 10(1), 91-98 (2016-12-13)
A four-step catalytic process was developed to produce p-methylstyrene from methylfuran, a biomass-derived species. First, methylfuran was acylated over zeolite H-Beta with acetic anhydride. Second, the acetyl group was reduced to an ethyl group with hydrogen over copper chromite. Third
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.