Alle Fotos(1)
Wichtige Dokumente
270253
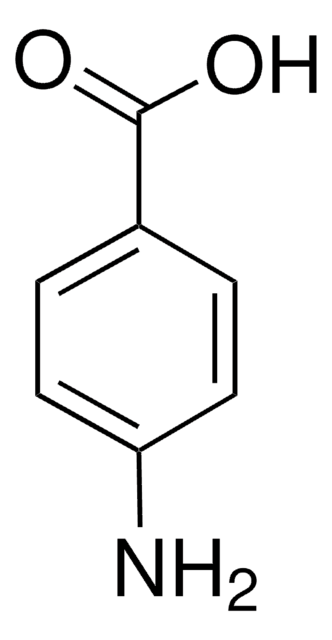
4-Hydroxybenzamid
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
HOC6H4CONH2
CAS-Nummer:
Molekulargewicht:
137.14
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
mp (Schmelzpunkt)
161-162 °C (lit.)
Funktionelle Gruppe
amide
SMILES String
NC(=O)c1ccc(O)cc1
InChI
1S/C7H7NO2/c8-7(10)5-1-3-6(9)4-2-5/h1-4,9H,(H2,8,10)
InChIKey
QXSAKPUBHTZHKW-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
The standard molar enthalpy of formation of 4-hydroxybenzamide was studied by micro- or macrocombustion calorimetry.
Anwendung
4-Hydroxybenzamide was used in the synthesis of balanol, a potent protein kinase C (PKC) inhibitor.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
R S Randad et al.
Bioorganic & medicinal chemistry, 4(9), 1471-1480 (1996-09-01)
A combination of structure-activity studies, kinetic analysis, X-ray crystallographic analysis, and modeling were employed in the design of a novel series of HIV-1 protease (HIV PR) inhibitors. The crystal structure of a complex of HIV PR with SRSS-2,5-bis[N-(tert-butyloxycarbonyl)amino]-3,4-dihydroxy-1, 6-diphenylhexane (1)
G D Hartman et al.
Bioorganic & medicinal chemistry letters, 9(6), 863-868 (1999-04-17)
A new series of potent, linearly-minimized, orally active, selective GPIIb/IIIa inhibitors is identified. Thus 15 (L-750,034) achieves interaction via a constrained, non-turned conformation that maintains the proper distance between its charged termini and full sulfonamide exosite interaction. The diminutive stature
C Emoto et al.
Xenobiotica; the fate of foreign compounds in biological systems, 37(12), 1408-1420 (2007-10-19)
CJ-036878, N-(3-phenethoxybenzyl)-4-hydroxybenzamide, was developed as an antagonist of the N-methyl-D-aspartate receptor NR2B subunit. Two dimeric metabolites, CJ-047710 and CJ-047713, were identified from the incubation mixture with CJ-036878 in human liver microsomes (HLM). The identification of the enzymes involved in the
Carlos E S Bernardes et al.
The journal of physical chemistry. A, 112(40), 10029-10039 (2008-09-13)
The energetics of the phenolic O-H bond in a series of 2- and 4-HOC 6H 4C(O)Y (Y = H, CH3, CH 2CH=CH2, C[triple bond]CH, CH2F, NH2, NHCH 3, NO2, OH, OCH3, OCN, CN, F, Cl, SH, and SCH3) compounds and
G D Smith et al.
Protein science : a publication of the Protein Society, 5(8), 1502-1511 (1996-08-01)
The structure of a symmetric T3R3f insulin hexamer, complexed with 4-hydroxybenzamide, has been determined using X-ray crystallographic techniques. Data were measured from six crystals grown in microgravity to a resolution of 1.4 A and the structure has been refined including
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.