254037
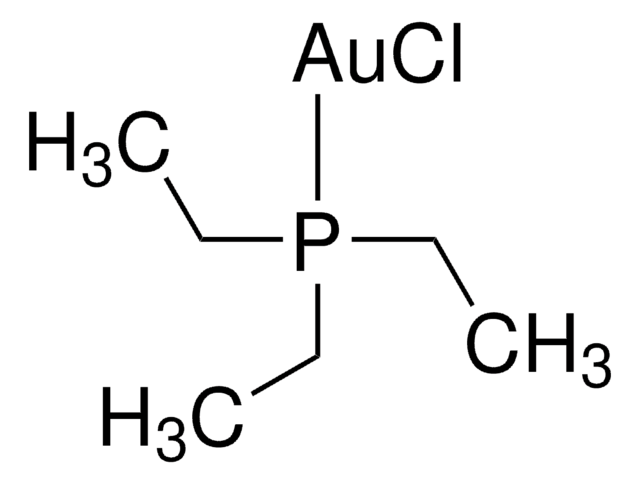
Chlor(triphenylphosphin)gold(I)
≥99.9% trace metals basis
Synonym(e):
Triphenylphosphingold(I)-chlorid
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥99.9% trace metals basis
Form
solid
Eignung der Reaktion
core: gold
reagent type: catalyst
SMILES String
Cl[Au].c1ccc(cc1)P(c2ccccc2)c3ccccc3
InChI
1S/C18H15P.Au.ClH/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;/h1-15H;;1H/q;+1;/p-1
InChIKey
IFPWCRBNZXUWGC-UHFFFAOYSA-M
Anwendung
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
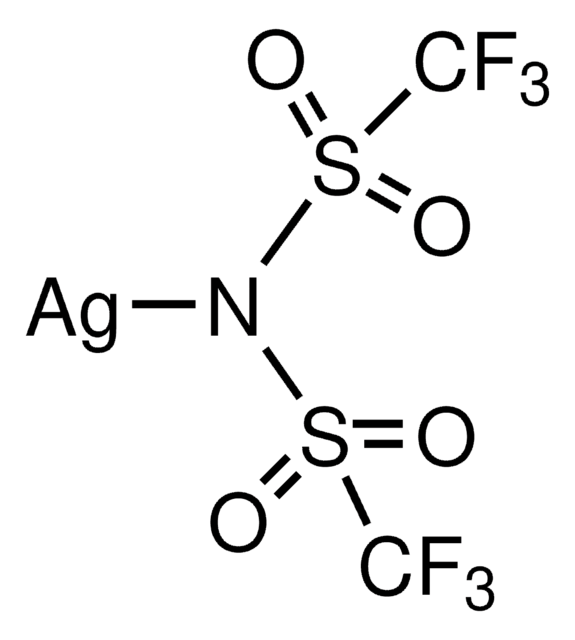
The importance of selectively fluorinating compounds in medicinal chemistry, biology, and organic synthesis is well appreciated and provides a major impetus to the discovery of new and mild fluorinating agents that can operate safely and efficiently.
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![Chloro[tris(para-trifluormethylphenyl)phosphin]gold(I) 99%](/deepweb/assets/sigmaaldrich/product/structures/250/453/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e/640/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e.png)
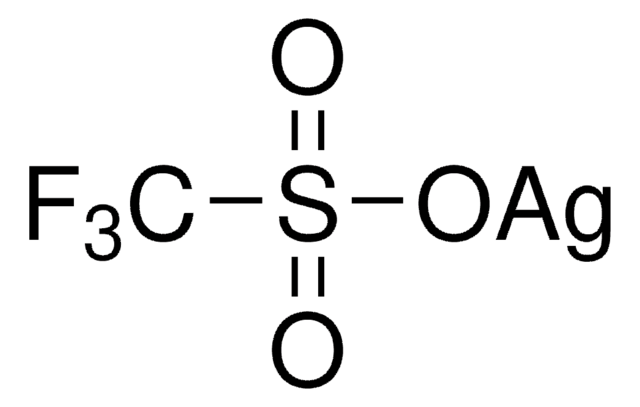
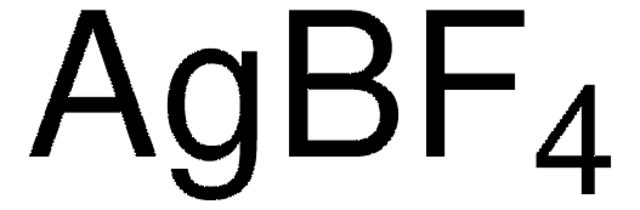

![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)
gold(I) (2:1) Toluol-Addukt](/deepweb/assets/sigmaaldrich/product/structures/104/897/81ee3e56-c988-4d0f-9614-1269b470316d/640/81ee3e56-c988-4d0f-9614-1269b470316d.png)

![Chloro[tris(2,4-di-tert-butylphenyl)phosphit]gold](/deepweb/assets/sigmaaldrich/product/structures/386/294/6df0db46-002b-4599-ad6c-451c419a3fc5/640/6df0db46-002b-4599-ad6c-451c419a3fc5.png)
![(Acetonitril)[(2-Biphenyl)di-tert-butylphosphin]gold(I)-Hexafluorantimonat](/deepweb/assets/sigmaaldrich/product/structures/216/222/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9/640/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9.png)