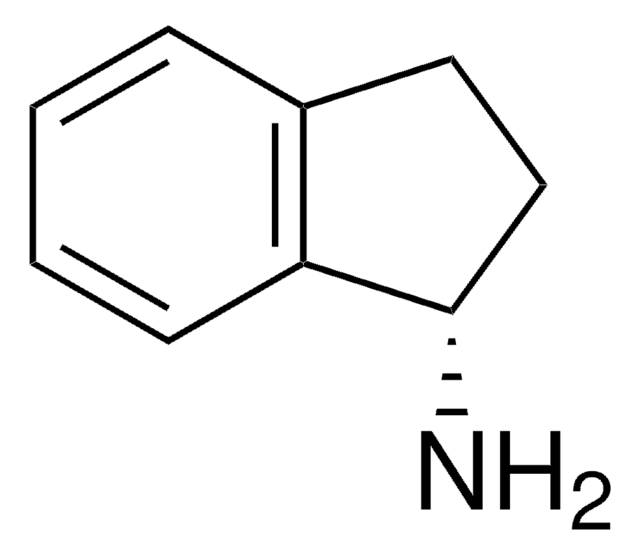
247820
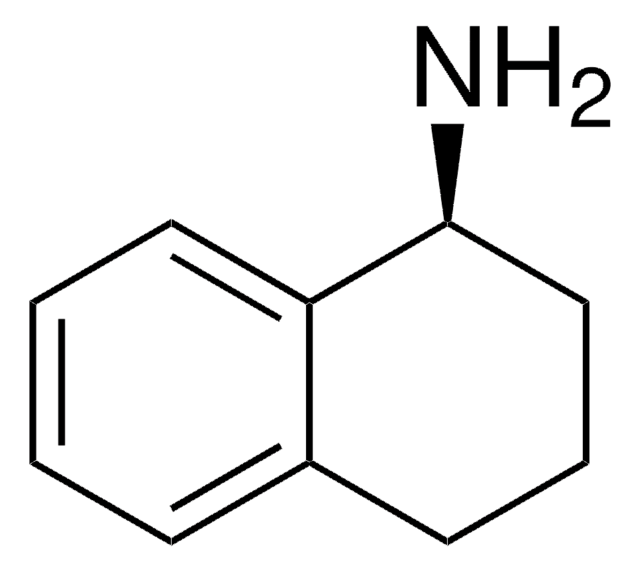
1,2,3,4-Tetrahydro-1-naphthylamin
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C10H13N
CAS-Nummer:
Molekulargewicht:
147.22
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
Form
liquid
Brechungsindex
n20/D 1.562 (lit.)
bp
246-247 °C/714 mmHg (lit.)
Dichte
1.026 g/mL at 25 °C (lit.)
SMILES String
NC1CCCc2ccccc12
InChI
1S/C10H13N/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,4,6,10H,3,5,7,11H2
InChIKey
JRZGPXSSNPTNMA-UHFFFAOYSA-N
Allgemeine Beschreibung
(R)-1,2,3,4-Tetrahydro-1-naphthylamine is an efficient reagent for iodocyclization of 4-aryl-4-pentenoic acids.
Anwendung
1,2,3,4-Tetrahydro-1-naphthylamine has been used in the preparation of new chiral phosphine-aminophosphine ligands.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Modular Phosphine-Aminophosphine Ligands Based on Chiral 1, 2, 3, 4-Tetrahydro-1-naphthylamine Backbone: A New Class of Practical Ligands for Enantioselective Hydrogenations.
Qiu M, et al.
Advanced Synthesis & Catalysis, 350(17), 2683-2689 (2008)
Jürgen Haas et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 11(19), 5777-5785 (2005-07-23)
Lactonizations are important steps in many synthetic sequences. Substrate-controlled reactions that use chiral auxiliaries or chiral alkenes have already been studied in depth. This study focuses on stereoselective reagent-controlled iodolactonizations, by application of a new method that uses complexes of
J S Shin et al.
Biotechnology and bioengineering, 73(3), 179-187 (2001-03-21)
A kinetic resolution process for the production of chiral amines was developed using an enzyme-membrane reactor (EMR) and a hollow-fiber membrane contactor with (S)-specific omega-transaminases (omega-TA) from Vibrio fluvialis JS17 and Bacillus thuringiensis JS64. The substrate solution containing racemic amine
Noelia Madroñal et al.
Nature communications, 7, 10923-10923 (2016-03-19)
The hippocampus is critical for the acquisition and retrieval of episodic and contextual memories. Lesions of the dentate gyrus, a principal input of the hippocampus, block memory acquisition, but it remains unclear whether this region also plays a role in
Effects of isomers of apomorphines on dopamine receptors in striatal and limbic tissue of rat brain.
N S Kula et al.
Life sciences, 37(11), 1051-1057 (1985-09-16)
The optical isomers of apomorphine (APO) and N-propylnorapomorphine (NPA) were interacted with three biochemical indices of dopamine (DA) receptors in extrapyramidal and limbic preparations of rat brain tissue. There were consistent isomeric preferences for the R(-) configuration of both DA
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.