Alle Fotos(1)
Wichtige Dokumente
183733
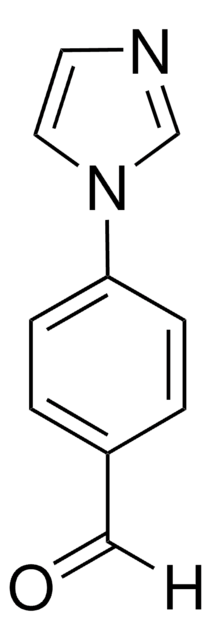
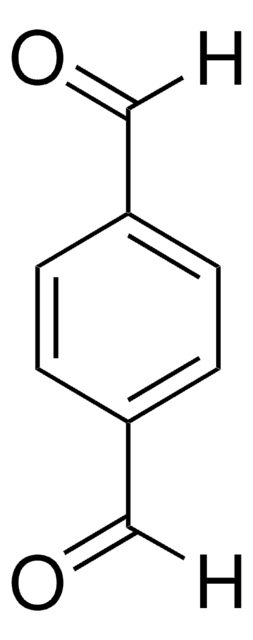
4′-(Imidazol-1-yl)acetophenon
96%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C11H10N2O
CAS-Nummer:
Molekulargewicht:
186.21
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352005
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
96%
Form
solid
mp (Schmelzpunkt)
108-110 °C (lit.)
Funktionelle Gruppe
ketone
SMILES String
CC(=O)c1ccc(cc1)-n2ccnc2
InChI
1S/C11H10N2O/c1-9(14)10-2-4-11(5-3-10)13-7-6-12-8-13/h2-8H,1H3
InChIKey
GAIQQJIMVVUTQN-UHFFFAOYSA-N
Allgemeine Beschreibung
4′-(Imidazol-1-yl)acetophenone is a selective thromboxane synthesis inhibitor.
Anwendung
4′-(Imidazol-1-yl)acetophenone was used in the synthesis of (R)-(+)-4′-(imidozol-1-yl)-phenyl ethanol using spiroborate catalyst.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
H D Uderman et al.
Prostaglandins, 24(2), 237-244 (1982-08-01)
The compound 4'-(imidazol-1-yl) acetophenone was demonstrated to be a selective thromboxane (Tx) synthetase inhibitor in spontaneously hypertensive rats (SHR). Serum TxB2 concentrations (from clotted blood) were suppressed by 89.1% (p less than 0.001) and 41.2% (p less than 0.01) at
W D Watkins et al.
Prostaglandins, 23(3), 273-285 (1982-03-01)
We assessed the effect of a specific thromboxane synthetase inhibitor (an imidazole derivative) on pulmonary hemodynamics and the concentrations of TxB2 (TxA2), 6-keto-PGF1 alpha (PGI2), and PGF2 in pulmonary lymph and transpulmonary blood samples following intravenous administration of E. coli
P A Craven et al.
The Journal of laboratory and clinical medicine, 116(4), 469-478 (1990-10-01)
Thromboxane contributes to the regulation of glomerular hemodynamics in experimental models of diabetes and has been implicated as mediator in some models of glomerular injury. In the present study we examined urinary albumin, protein, and thromboxane B2 (TXB2) excretion during
Viatcheslav Stepanenko et al.
Tetrahedron, asymmetry, 18(23), 2738-2745 (2007-11-26)
The effectiveness of several spiroborate ester catalysts was investigated in the asymmetric borane reduction of 2-, 3-, 4-acetylpyridines under different reaction conditions. Highly enantiomerically enriched 1-(2-, 3- and 4-pyridyl)ethanols and 1-(heterocyclic)ethanols were obtained using 1 to 10% catalytic loads of
J Triscari et al.
International journal of obesity, 11 Suppl 3, 43-51 (1987-01-01)
A selective inhibitor of thromboxane synthase, Ro 22-3581 has been shown to be a useful tool for investigating the relationship between hyperinsulinemia and obesity. These studies have established that the pharmacologic normalization of the hyperinsulinemia associated with elevated weights in
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.