149136
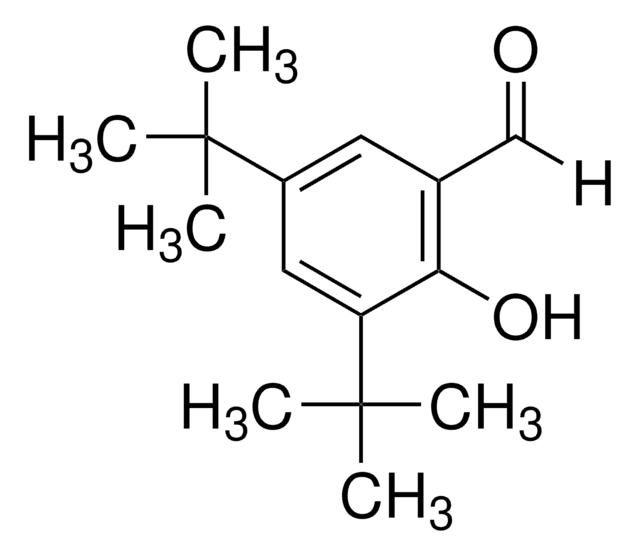
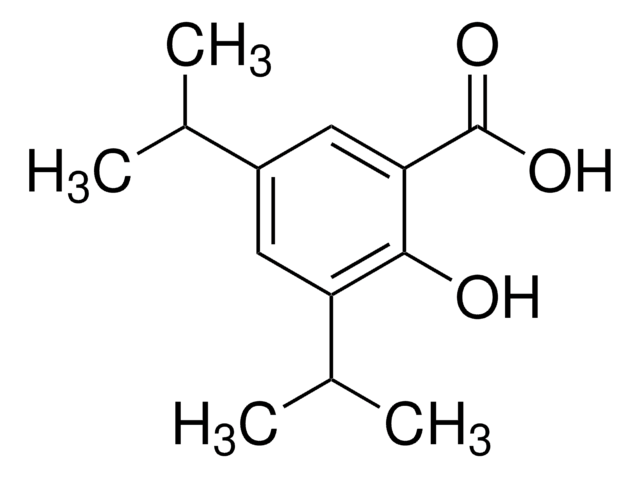
3,5-Di-tert-butylsalicylsäure
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
[(CH3)3C]2C6H2-2-(OH)CO2H
CAS-Nummer:
Molekulargewicht:
250.33
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
mp (Schmelzpunkt)
157-162 °C (lit.)
Funktionelle Gruppe
carboxylic acid
SMILES String
CC(C)(C)c1cc(C(O)=O)c(O)c(c1)C(C)(C)C
InChI
1S/C15H22O3/c1-14(2,3)9-7-10(13(17)18)12(16)11(8-9)15(4,5)6/h7-8,16H,1-6H3,(H,17,18)
InChIKey
ZWQBZEFLFSFEOS-UHFFFAOYSA-N
Anwendung
3,5-Di-tert-butylsalicylic acid was used to study long wavelength fluorescence emission of 3,5-Di-tert-butylsalicylic acid in a variety of organic solvents. It was also used to catalyze the reaction between aldehydes and silyl ketene acetals.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
354.2 °F - closed cup
Flammpunkt (°C)
179 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Veli T Kasumov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 107, 31-38 (2013-02-19)
A series of new polyfluorinated palladium(II) complexes (7-12) of N-polyfluorophenyl-3,5-di-tert-butylsalicylaldimines (1-6) have been synthesized. They were characterized by analytical, spectroscopic (UV/Vis, IR, (1)H NMR, and ESR), electrochemical methods and their chemical oxidation and hydrogenation catalytic activity were studied. The X-ray
Photoinduced proton transfers in 3, 5-di-tert-butylsalicylic acid.
The Journal of Physical Chemistry, 99(32), 12103-12108 (1995)
A first example of macromolecular Ti (IV) Lewis acid in the catalytic enantioselective Mukaiyama reaction.
Tetrahedron Asymmetry, 9(9), 1479-1482 (1998)
M V Chidambaram et al.
Journal of pharmaceutical sciences, 80(8), 810-811 (1991-08-01)
The initial yield of 3,5-di-t-butylsalicylic acid obtained via Kolbe-Schmitt carboxylation of the potassium salt of 2,4-di-t-butylphenol was less than 1% and was accompanied by a 65% yield of 2,2'-dihydroxy-3,3',5,5'- tetra-t-butylbiphenyl, a dimer of the 2,4-di-t-butylphenol formed by ortho coupling of
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.