Alle Fotos(3)
Wichtige Dokumente
116416
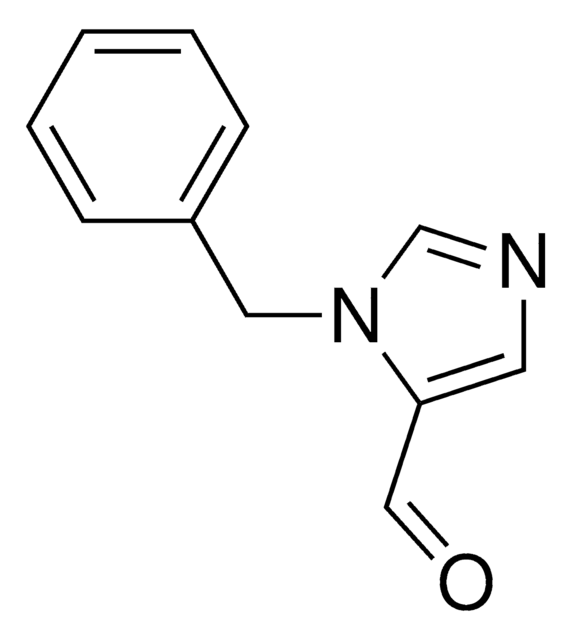
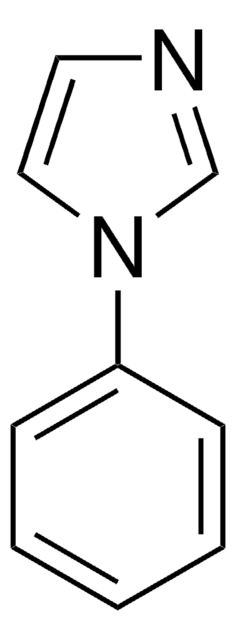
1-Benzylimidazol
99%
Synonym(e):
1-(Phenylmethyl)-1H-imidazole, 1-Benzyl-1H-imidazole, N-Benzylimidazole
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C10H10N2
CAS-Nummer:
Molekulargewicht:
158.20
Beilstein:
114571
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352005
eCl@ss:
39161001
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
bp
310 °C (lit.)
mp (Schmelzpunkt)
68-70 °C (lit.)
Funktionelle Gruppe
phenyl
SMILES String
C(c1ccccc1)n2ccnc2
InChI
1S/C10H10N2/c1-2-4-10(5-3-1)8-12-7-6-11-9-12/h1-7,9H,8H2
InChIKey
KKKDZZRICRFGSD-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
1-Benzylimidazole has been used to prepare cyclodextrin-ionic liquid polymer (βCD-BIMOTs-TDI).
Biochem./physiol. Wirkung
1-Benzylimidazole is a CYP inhibitor that inhibits the biotransformation of MeO-BDEs (methoxylated-brominated diphenyl ethers) to OH-BDEs (hydroxylated) in fishes.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Jérémie Doiron et al.
European journal of medicinal chemistry, 46(9), 4010-4024 (2011-06-28)
A series of bis- and mono-benzonitrile or phenyl analogues of letrozole 1, bearing (1,2,3 and 1,2,5)-triazole or imidazole, were synthesized and screened for their anti-aromatase activities. The unsubstituted 1,2,3-triazole 10a derivative displayed inhibitory activity comparable with that of the aromatase
José María Navas et al.
Environmental toxicology and chemistry, 22(4), 830-836 (2003-04-11)
Xenobiotics can induce cytochrome P4501A (CYP1A) by ligand binding to the aryl hydrocarbon receptor (AhR). Typical AhR ligands are polycyclic aromatic compounds with planar molecular conformation. The present work investigated the ability of the N-imidazole derivative, 1-benzylimidazole (BIM), to induce
P Rothenbach et al.
Journal of applied physiology (Bethesda, Md. : 1985), 83(2), 530-536 (1997-08-01)
This study examines the hypothesis that intestinal ischemia-reperfusion (I/R) injury contributes to renal dysfunction by altered renal eicosanoid release. Anesthetized Sprague-Dawley rats underwent 60 min of sham or superior mesenteric artery (SMA) occlusion with 60 min of reperfusion. The I/R
A Grothusen et al.
Archives of toxicology, 71(1-2), 64-71 (1996-01-01)
Liver microsomes are a frequently used probe to investigate the phase I metabolism of xenobiotics in vitro. Structures containing nucleophilic hetero-atoms are possible substrates for cytochrome P450 enzymes (P450) and flavin-containing monooxygenases (FMO). Both enzymes are located in the endoplasmatic
Fredrik Jernerén et al.
Lipids, 47(7), 707-717 (2012-05-01)
(8R)-Hydroperoxy-(9Z,12Z)-octadecadienoic acid (8-HPODE) is formed by aspergilli as an intermediate in biosynthesis of oxylipins with effects on sporulation. 8-HPODE is transformed by separate diol synthases to (5S,8R)-dihydroxy- and (8R,11S)-dihydroxy-(9Z,12Z)-octadecadienoic acids (5,8- and 8,11-DiHODE). The former is formed by the cytochrome
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)