S3626
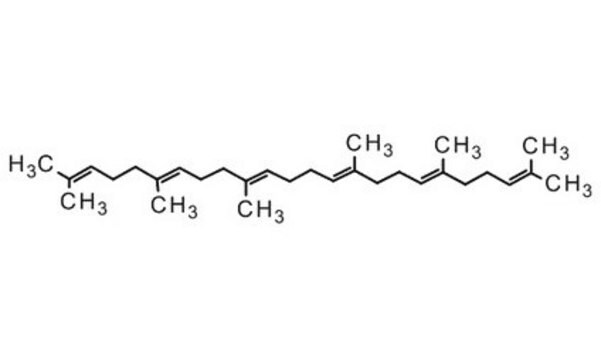
Squalene
≥98%, liquid
Sinónimos:
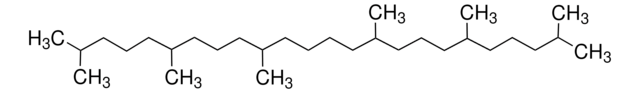
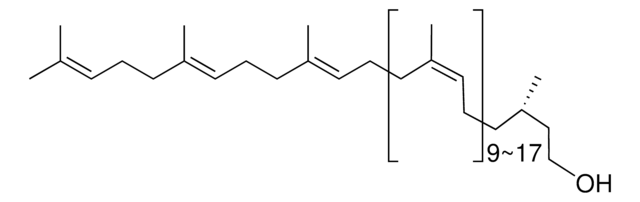
2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene
About This Item
Productos recomendados
Quality Level
assay
≥98%
form
liquid
color
light yellow
refractive index
n20/D 1.494 (lit.)
bp
285 °C/25 mmHg (lit.)
mp
−75 °C (lit.)
density
0.858 g/mL at 25 °C (lit.)
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
CC(C)=CCCC(C)=CCCC(C)=CCC\C=C(/C)CCC=C(C)CCC=C(C)C
InChI
1S/C30H50/c1-25(2)15-11-19-29(7)23-13-21-27(5)17-9-10-18-28(6)22-14-24-30(8)20-12-16-26(3)4/h15-18,23-24H,9-14,19-22H2,1-8H3/b27-17+,28-18+,29-23+,30-24+
InChI key
YYGNTYWPHWGJRM-AAJYLUCBSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- as a standard for lipid identification and quantification
- in the isolation of macrophages for parasite incubation
- as a standard for the quantification of squalene in squalene analysis of oil samples
Biochem/physiol Actions
signalword
Danger
hcodes
Hazard Classifications
Asp. Tox. 1
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Biosynthesis of cholesterol generally takes place in the endoplasmic reticulum of hepatic cells and begins with acetyl- CoA, which is mainly derived from an oxidation reaction in the mitochondria. Acetyl-CoA and acetoacetyl-CoA are converted to 3-hydroxy- 3-methylglutaryl-CoA (HMG-CoA) by HMG-CoA synthase.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico