P8129
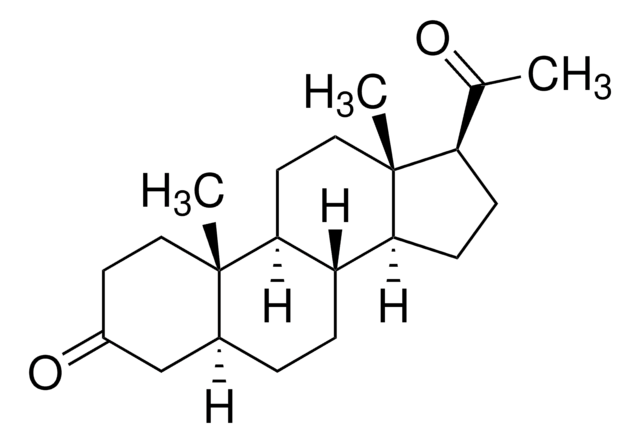
5β-Pregnan-3α-ol-20-one
Sinónimos:
3α-Hydroxy-5β-pregnan-20-one, 3α-Hydroxy-5β-tetrahydroprogesterone, Pregnanolone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C21H34O2
Número de CAS:
Peso molecular:
318.49
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
biological source:
synthetic (organic)
form:
powder
assay:
≥98% (TLC)
Productos recomendados
biological source
synthetic (organic)
assay
≥98% (TLC)
form
powder
solubility
chloroform: 50 mg/mL, clear, colorless
shipped in
ambient
storage temp.
room temp
SMILES string
[H]C12CCC3C4CCC(C(C)=O)C4(C)CCC3C1(C)CC[C@H](O)C2
InChI
1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3
InChI key
AURFZBICLPNKBZ-UHFFFAOYSA-N
Gene Information
rat ... Gabra2(29706)
Biochem/physiol Actions
5β-Pregnan-3α-ol-20-one or pregnanolone is a neurosteroid that acts as a positive allosteric modulator (PAMs) of γ-aminobutyric acid A receptors (GABAARs). It mediates anti-convulsive, anxiolytic and sedative effects. Pregnanolone inhibits γ-aminobutyric acid C receptor (GABAC) receptor-based response and favors GABAA receptor-mediated currents.
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
P Li et al.
British journal of pharmacology, 171(23), 5446-5457 (2014-08-15)
Neurosteroids potentiate responses of the GABAA receptor to the endogenous agonist GABA. Here, we examined the ability of neurosteroids to potentiate responses to the allosteric activators etomidate, pentobarbital and propofol. Electrophysiological assays were conducted on rat α1β2γ2L GABAA receptors expressed
Selwyn S Jayakar et al.
The Journal of biological chemistry, 295(33), 11495-11512 (2020-06-17)
Allopregnanolone (3α5α-P), pregnanolone, and their synthetic derivatives are potent positive allosteric modulators (PAMs) of GABAA receptors (GABAARs) with in vivo anesthetic, anxiolytic, and anti-convulsant effects. Mutational analysis, photoaffinity labeling, and structural studies have provided evidence for intersubunit and intrasubunit steroid-binding
Eva Innala et al.
Acta obstetricia et gynecologica Scandinavica, 91(12), 1445-1452 (2012-08-29)
To measure serum concentrations of progesterone, estradiol and 5α- and 5β-reduced progesterone metabolites in the follicular and luteal phases of the menstrual cycle in women with latent acute intermittent porphyria and manifest acute intermittent porphyria in comparison with healthy control
Jane Evans et al.
Neuropharmacology, 63(8), 1315-1326 (2012-09-04)
Chronic stress has been implicated as a causal factor in depression and anxiety, and is associated with neuroendocrine dysfunction and impaired hippocampal neurogenesis. The neurosteroid allopregnanolone (3α,5α-THP; ALLO) has been shown to be reduced in depressed patients. ALLO is "stress
Barbora Slavíková et al.
Journal of medicinal chemistry, 56(6), 2323-2336 (2013-02-21)
(25R)-3β-Hydroxy-5α-spirostan-12-one (hecogenin) and 11α-hydroxypregn-4-ene-3,20-dione (11α-hydroxyprogesterone) were used as starting materials for the synthesis of a series of 11- and 12-substituted derivatives of 5ξ-pregnanolone (3α-hydroxy-5α-pregnan-20-one and 3α-hydroxy-5β-pregnan-20-one), the principal neurosteroid acting via γ-aminobutyric acid (GABA). These analogues were designed to study
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico