A4330
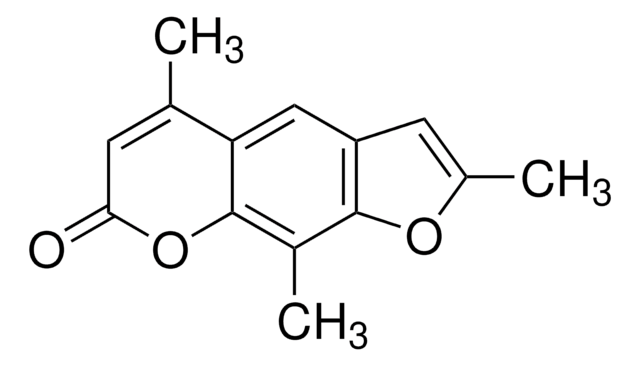
4′-Aminomethyltrioxsalen hydrochloride
Sinónimos:
4′-Aminomethyl-4,5′,8-trimethylpsoralen hydrochloride
About This Item
Productos recomendados
form
powder
Quality Level
solubility
H2O: 1 mg/mL
DMSO: 2 mg/mL
storage temp.
2-8°C
SMILES string
CC1=CC(=O)Oc2c(C)c3oc(C)c(CN)c3cc12
InChI
1S/C15H15NO3/c1-7-4-13(17)19-14-8(2)15-11(5-10(7)14)12(6-16)9(3)18-15/h4-5H,6,16H2,1-3H3
InChI key
WBIICVGYYRRURR-UHFFFAOYSA-N
Application
Biochem/physiol Actions
Features and Benefits
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Contenido relacionado
n proliferating cells, the cell cycle consists of four phases. Gap 1 (G1) is the interval between mitosis and DNA replication that is characterized by cell growth. Replication of DNA occurs during the synthesis (S) phase, which is followed by a second gap phase (G2) during which growth and preparation for cell division occurs. Together, these three stages comprise the interphase phase of the cell cycle. Interphase is followed by the mitotic (M) phase.
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico