92549
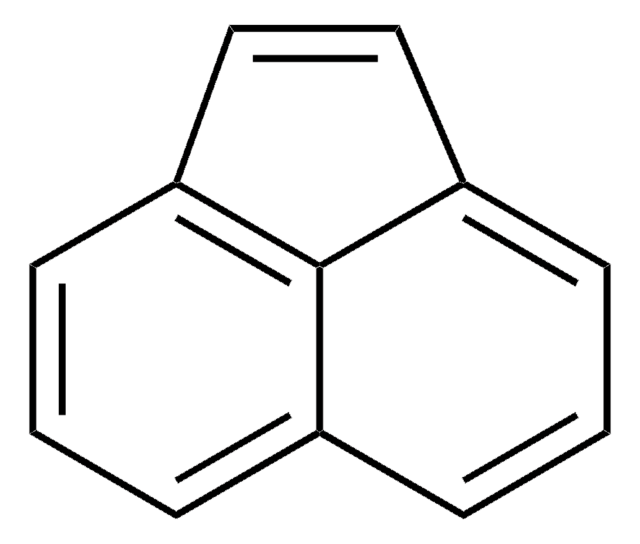
Acenaphthylene
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
About This Item
Productos recomendados
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
280 °C (lit.)
mp
78-82 °C (lit.)
89-92 °C
density
0.899 g/mL at 25 °C (lit.)
application(s)
environmental
format
neat
storage temp.
2-8°C
SMILES string
c1cc2C=Cc3cccc(c1)c23
InChI
1S/C12H8/c1-3-9-4-2-6-11-8-7-10(5-1)12(9)11/h1-8H
InChI key
HXGDTGSAIMULJN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com.
Application
Packaging
Legal Information
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
251.6 °F - closed cup
flash_point_c
122.0 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Protocolos
US EPA Method 8270 (PAH only): GC Analysis of PAHs on SLB®-5ms
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico