81831
Propargyl bromide solution
purum, ~80% in toluene
Sinónimos:
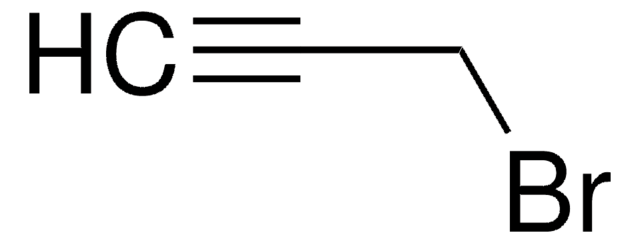
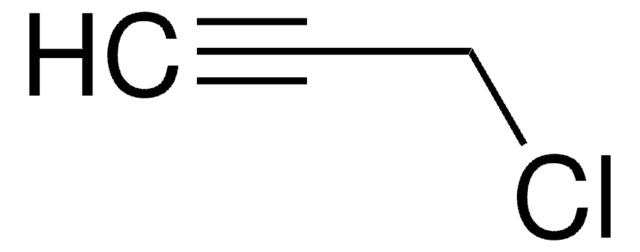
3-Bromo-1-propyne
About This Item
Productos recomendados
grade
purum
Quality Level
form
liquid
contains
~0.3% magnesium oxide light as stabilizer
concentration
~80% in toluene
density
1.39 g/mL at 20 °C
functional group
alkyl halide
bromo
storage temp.
2-8°C
SMILES string
BrCC#C
InChI
1S/C3H3Br/c1-2-3-4/h1H,3H2
InChI key
YORCIIVHUBAYBQ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- new arabinogalactan propargyl ethers with degree of substitution (DS) up to 2.8, by propargylation of arabino-3,6-galactan
- conjugated polyelectrolyte with polyacetylene as the backbone and pyridinium as side groups, poly(propargyl pyridinium bromide)
- benzodiazepine derivative, 4-phenyl-1-(prop-2-yn-1-yl)-1H-1,5-benzodiazepin-2(3H)-one
- N(3)-propargylated 2′-deoxyuridine, which can be encorporated in the DNA
- chiral oxygenated acyclic natural products, via distereoselective propargylation of α-hydroxy aldehydes
signalword
Danger
Hazard Classifications
Acute Tox. 3 Oral - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 2 - STOT SE 3
target_organs
Central nervous system, Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
64.4 °F - closed cup
flash_point_c
18 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico