30039
p-Cymene
analytical standard
Sinónimos:
1-Isopropyl-4-methylbenzene, 4-Isopropyltoluene
About This Item
Productos recomendados
grade
analytical standard
Quality Level
vapor density
4.62 (vs air)
vapor pressure
1.5 mmHg ( 20 °C)
3.7 mmHg ( 37.7 °C)
assay
≥99.5% (GC)
autoignition temp.
817 °F
shelf life
limited shelf life, expiry date on the label
expl. lim.
5.6 %
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.490 (lit.)
n20/D 1.491
bp
176-178 °C (lit.)
density
0.86 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
format
neat
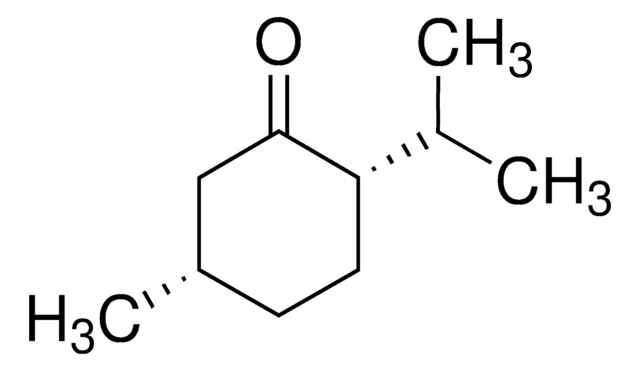
SMILES string
CC(C)c1ccc(C)cc1
InChI
1S/C10H14/c1-8(2)10-6-4-9(3)5-7-10/h4-8H,1-3H3
InChI key
HFPZCAJZSCWRBC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Other Notes
Recommended products
signalword
Danger
Hazard Classifications
Acute Tox. 3 Inhalation - Aquatic Chronic 2 - Asp. Tox. 1 - Flam. Liq. 3 - Repr. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
125.6 °F - closed cup
flash_point_c
52 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocolos
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico