A55500
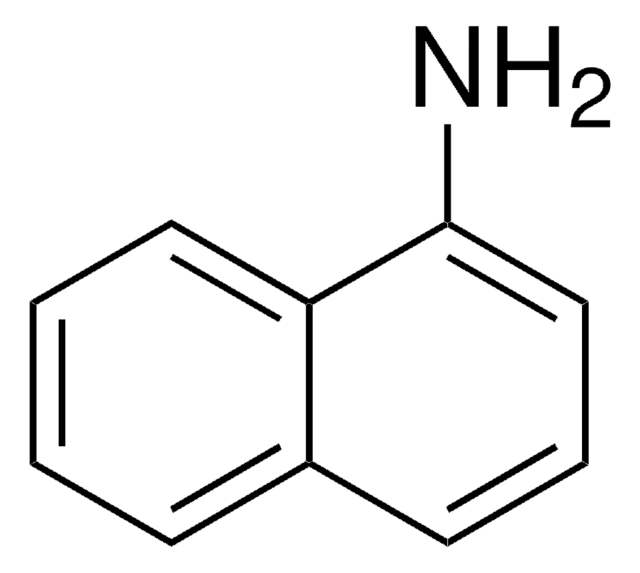
2-Aminofluorene
98%
Sinónimos:
2-Fluorenamine, 2-Fluorenylamine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C13H11N
Número de CAS:
Peso molecular:
181.23
Beilstein/REAXYS Number:
1945861
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
powder
mp
124-128 °C (lit.)
SMILES string
Nc1ccc-2c(Cc3ccccc-23)c1
InChI
1S/C13H11N/c14-11-5-6-13-10(8-11)7-9-3-1-2-4-12(9)13/h1-6,8H,7,14H2
InChI key
CFRFHWQYWJMEJN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 2 - Carc. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
R H Heflich et al.
Mutation research, 318(2), 73-114 (1994-10-01)
2-Acetylaminofluorene and 2-aminofluorene are among the most intensively studied of all chemical mutagens and carcinogens. Fundamental research findings concerning the metabolism of 2-acetylaminofluorene to electrophilic derivatives, the interaction of these derivatives with DNA, and the carcinogenic and mutagenic responses that
Stephen W Holman et al.
Rapid communications in mass spectrometry : RCM, 22(15), 2355-2365 (2008-07-10)
A 50 m/z unit loss from protonated 4-benzenesulfinyl-3-methylphenylamine has been observed and investigated using electrospray ionisation quadrupole ion trap mass spectrometry (ESI-QIT-MS). It was hypothesised that the specific fragmentation was affected by the presence of an ortho methyl group in
Dominique Y Burnouf et al.
Journal of molecular biology, 386(4), 951-961 (2009-01-20)
The model carcinogen N-2-acetylaminofluorene covalently binds to the C8 position of guanine to form two adducts, the N-(2'-deoxyguanosine-8-yl)-aminofluorene (G-AF) and the N-2-(2'-deoxyguanosine-8-yl)-acetylaminofluorene (G-AAF). Although they are chemically closely related, their biological effects are strongly different and they are processed by
Jun Zhao et al.
Stem cell research & therapy, 10(1), 165-165 (2019-06-15)
Mounting evidence has shown that a novel subset of mesenchymal stem cells (MSCs) derived from human gingiva referred to as gingival mesenchymal stem cells (GMSCs) displays a greater immunotherapeutic potential and regenerative repair expression than MSCs obtained from other tissues.
Olga Rechkoblit et al.
Nature structural & molecular biology, 17(3), 379-388 (2010-02-16)
The aromatic amine carcinogen 2-aminofluorene (AF) forms covalent adducts with DNA, predominantly with guanine at the C8 position. Such lesions are bypassed by Y-family polymerases such as Dpo4 via error-free and error-prone mechanisms. We show that Dpo4 catalyzes elongation from
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico