798436
Endo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine
Sinónimos:
(1S,4S,5S)-5-(naphthalen-1-yl)-2-tosyl-2-aza-5-phosphabicyclo[2.2.1]heptane
About This Item
Productos recomendados
form
powder
Quality Level
storage condition
under inert gas
functional group
phosphine
sulfonamide
storage temp.
2-8°C
SMILES string
O=S(N1C[C@H]2[P@@](C3=CC=CC4=C3C=CC=C4)C[C@@H]1C2)(C5=CC=C(C)C=C5)=O
InChI
1S/C22H22NO2PS/c1-16-9-11-20(12-10-16)27(24,25)23-14-19-13-18(23)15-26(19)22-8-4-6-17-5-2-3-7-21(17)22/h2-12,18-19H,13-15H2,1H3
InChI key
RGHUOMJULXTHPX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
Other Notes
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
related product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![Exo-2-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/324/907/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4/640/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4.png)
![Endo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/060/39f2b621-f484-49c3-b692-cdb610e8c517/640/39f2b621-f484-49c3-b692-cdb610e8c517.png)
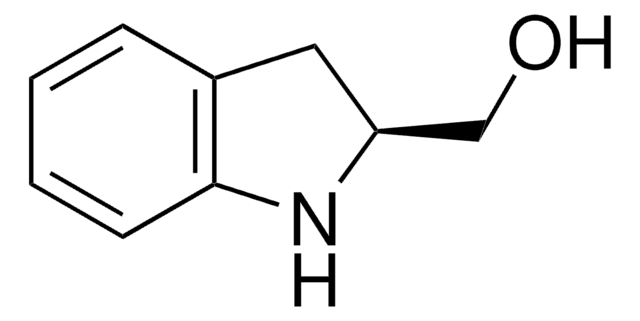
![Exo-4-anisole Kwon [2.2.1] bicyclic phosphine](/deepweb/assets/sigmaaldrich/product/structures/114/753/2a544671-b0e0-4556-8dc3-46c126d6c8ab/640/2a544671-b0e0-4556-8dc3-46c126d6c8ab.png)
![Exo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![Calix[4]arene-25,26,27,28-tetrol 95%](/deepweb/assets/sigmaaldrich/product/structures/198/765/9972559b-b50f-4745-b1fd-9a468e47ef55/640/9972559b-b50f-4745-b1fd-9a468e47ef55.png)
![(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)
![Tris[3-(trimethoxysilyl)propyl] isocyanurate technical grade](/deepweb/assets/sigmaaldrich/product/structures/239/690/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf/640/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf.png)
![Endo-4-Methoxyphenyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/404/012/38bdf2c6-e120-483d-8c3c-8fa3b328963c/640/38bdf2c6-e120-483d-8c3c-8fa3b328963c.png)

