682144
(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane
96%
Sinónimos:
(R)-Phanephos
About This Item
Productos recomendados
Quality Level
assay
96%
form
solid
optical activity
[α]/D -34±4°, c = 1 in chloroform
mp
222-226 °C
functional group
phosphine
InChI
1S/C40H34P2/c1-5-13-35(14-6-1)41(36-15-7-2-8-16-36)39-29-31-21-25-33(39)27-23-32-22-26-34(28-24-31)40(30-32)42(37-17-9-3-10-18-37)38-19-11-4-12-20-38/h1-22,25-26,29-30H,23-24,27-28H2
InChI key
GYZZZILPVUYAFJ-UHFFFAOYSA-N
Application
- Enantioselective reductive cyclization of 1,6-enynes via asymmetric hydrogenation in the presence of a rhodium catalyst to form alkylidene-substituted heterocycles.
- Asymmetric hydroboration of 3,3-disubstituted cyclopropenes to form 2,2-disubstituted cyclopropyl boronates.
- Asymmetric ring-opening reactions of azabenzonorbornadienes in the presence of zinc(II) triflate and palladium(II) acetate to form aminodihydronaphthalenes.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
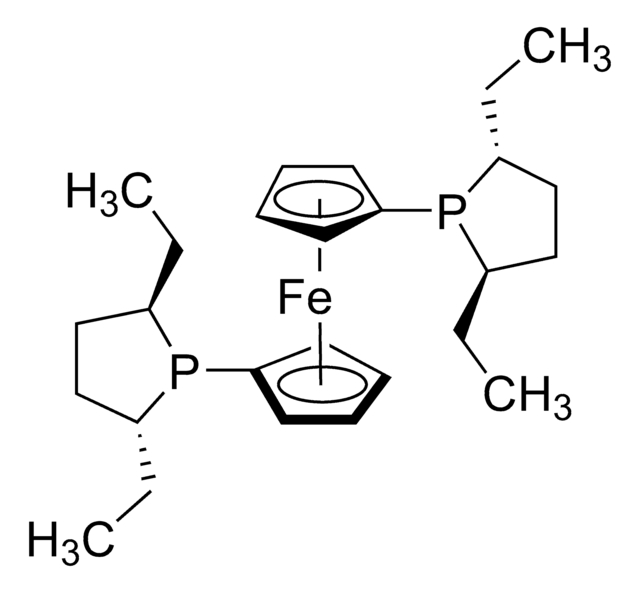
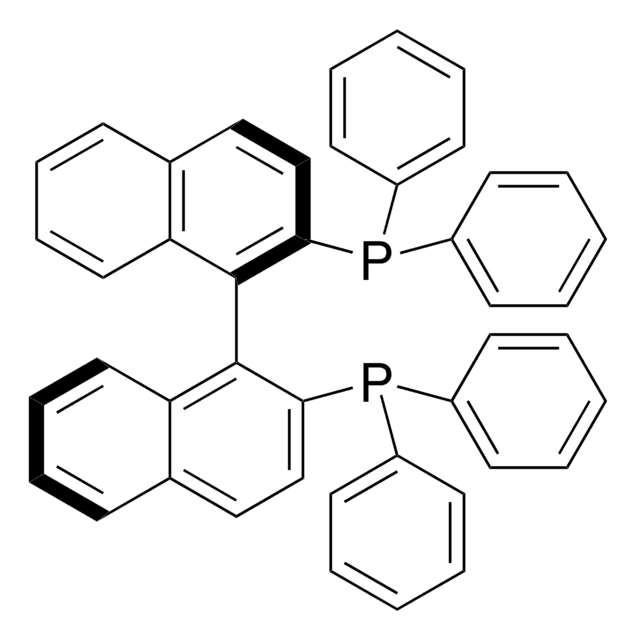
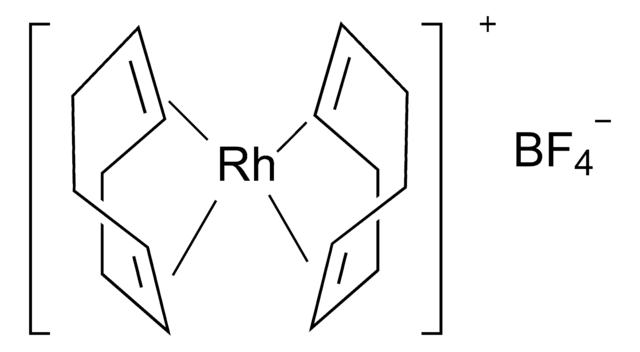
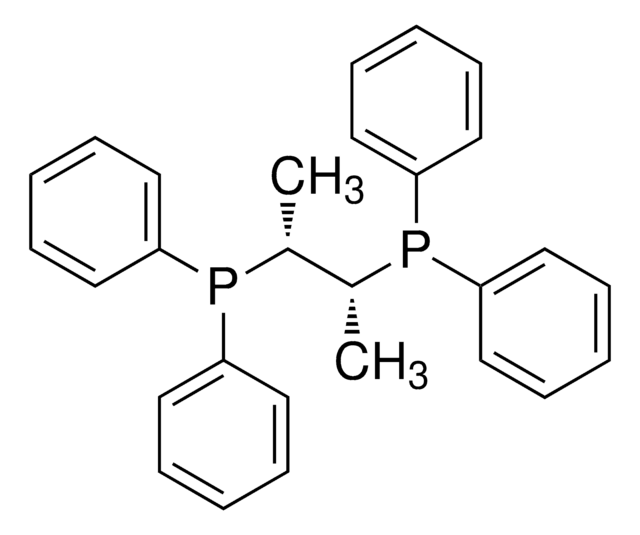
Los clientes también vieron
Artículos
The P-Phos ligand family was developed by Professor Chan of Hong Kong Polytechnic University and licensed to JM CCT in 2002. P-Phos is an atropisomeric biaryl bisphosphine with the unique feature of incorporating two methoxy-substituted pyridine rings in the backbone.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![(S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/396/009/d814b698-3227-4aef-b415-cb5f5730aa13/640/d814b698-3227-4aef-b415-cb5f5730aa13.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)
