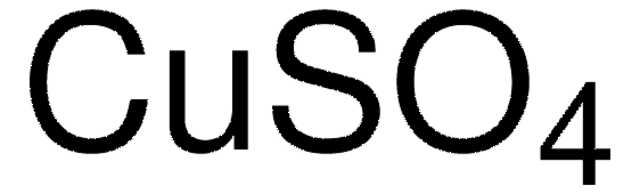678937
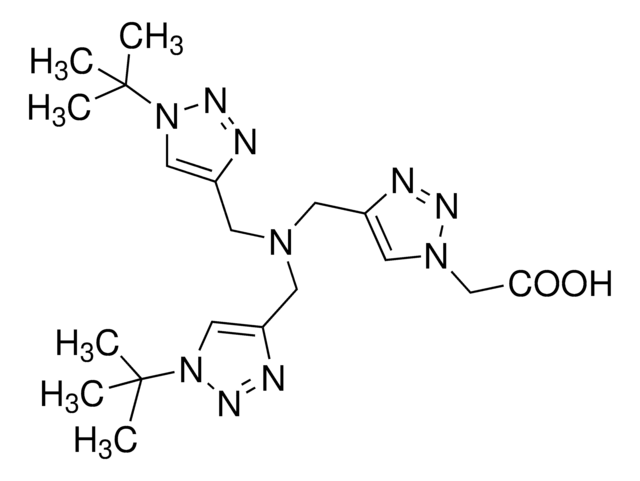
Tris[(1-benzyl-1H-1, 2, 3-triazol-4-yl)methyl]amine
97%
Sinónimos:
TBTA
About This Item
Productos recomendados
Quality Level
assay
97%
form
solid
reaction suitability
reagent type: ligand
reaction type: click chemistry
mp
132-143 °C
storage temp.
−20°C
SMILES string
C(N(Cc1cn(Cc2ccccc2)nn1)Cc3cn(Cc4ccccc4)nn3)c5cn(Cc6ccccc6)nn5
InChI
1S/C30H30N10/c1-4-10-25(11-5-1)16-38-22-28(31-34-38)19-37(20-29-23-39(35-32-29)17-26-12-6-2-7-13-26)21-30-24-40(36-33-30)18-27-14-8-3-9-15-27/h1-15,22-24H,16-21H2
InChI key
WKGZJBVXZWCZQC-UHFFFAOYSA-N
Categorías relacionadas
Application
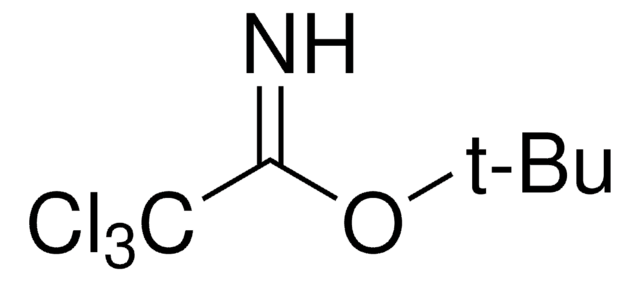
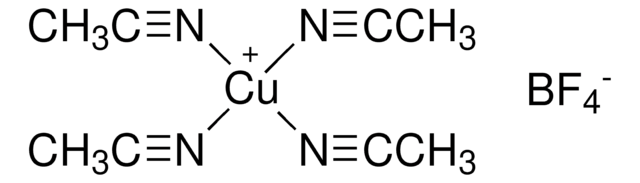
Related product
hcodes
Hazard Classifications
Aquatic Chronic 4
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| 678937-1G | |
| 678937-250MG | |
| 678937-500MG | 4061833499726 |
| 678937-50MG | 4061832749372 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico