238082
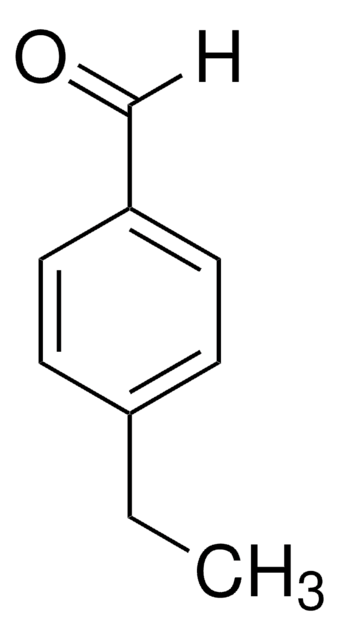
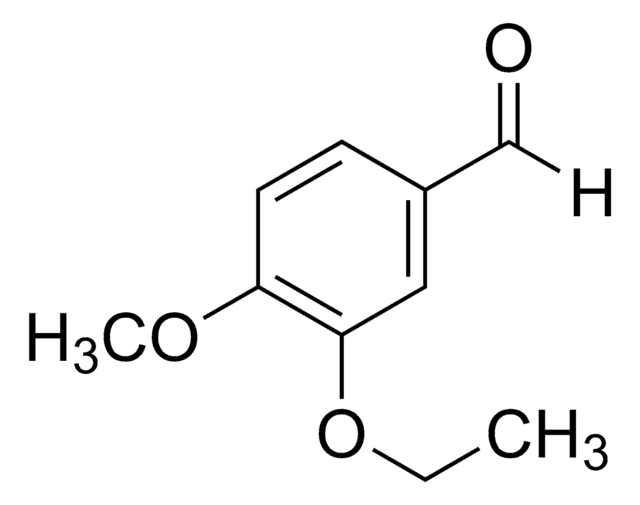
4-Butoxybenzaldehyde
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3(CH2)3OC6H4CHO
Número de CAS:
Peso molecular:
178.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.539 (lit.)
bp
285 °C (lit.)
density
1.031 g/mL at 25 °C (lit.)
functional group
aldehyde
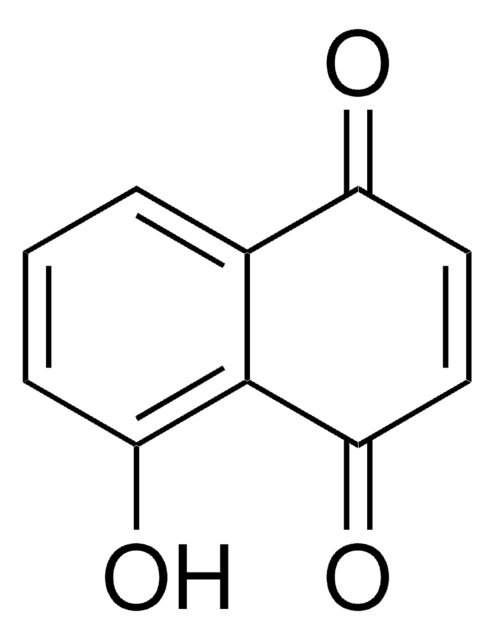
SMILES string
CCCCOc1ccc(C=O)cc1
InChI
1S/C11H14O2/c1-2-3-8-13-11-6-4-10(9-12)5-7-11/h4-7,9H,2-3,8H2,1H3
InChI key
XHWMNHADTZZHGI-UHFFFAOYSA-N
Categorías relacionadas
General description
Kinetic constant for the inhibition of the diphenolase activity of mushroom tyrosinase by 4-butoxybenzaldehyde has been evaluated.
Application
4-Butoxybenzaldehyde has been used in the synthesis of:
- 6-amino-4-(4-butoxyphenyl)-3,5-dicyanopyridine-2(1H)-thione
- 16-(p-butoxybenzylidene)androsta-1,4-diene-3,17-dione via condensation reaction with androsta-1,4-diene-3,17-dione
Legal Information
Darkens in storage with no loss in purity
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
>235.4 °F - closed cup
flash_point_c
> 113 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
16-(p-Butoxybenzylidene) androsta-1, 4-diene-3, 17-dione.
Ogawa K, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(7), 1359-1361 (1992)
Michael reaction in synthesis of 6-amino-4-(4-butoxyphenyl)-3, 5-dicyanopyridine-2 (1H)-thione.
Dyachenko VD and Litvinov VP.
Chemistry of Heterocyclic Compounds, 34(2), 188-194 (1998)
Dalila Rocco et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-12)
The preparation of 24-functionalized 12,22:26,32-terpyridines (4'-functionalized 3,2:6',3''-terpyridines) by the reaction of three 4-alkoxybenzaldehydes with 3-acetylpyridine and ammonia was investigated; under identical reaction conditions, two (R = nC4H9, C2H5) gave the expected products whereas a third (R = nC3H7) gave only
M Jiménez et al.
Journal of agricultural and food chemistry, 49(8), 4060-4063 (2001-08-22)
A kinetic study of the inhibition of mushroom tyrosinase by 4-substituted benzaldehydes showed that these compounds behave as classical competitive inhibitors, inhibiting the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) by mushroom tyrosinase (o-diphenolase activity). The kinetic parameter (K(I)) characterizing this inhibition was
Naoko Ueno et al.
Langmuir : the ACS journal of surfaces and colloids, 33(22), 5393-5397 (2017-05-16)
We evaluated the speed profile of self-propelled underwater oil droplets comprising a hydrophobic aldehyde derivative in terms of their diameter and the surrounding surfactant concentration using a microfluidic device. We found that the speed of the oil droplets is dependent
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico