579920
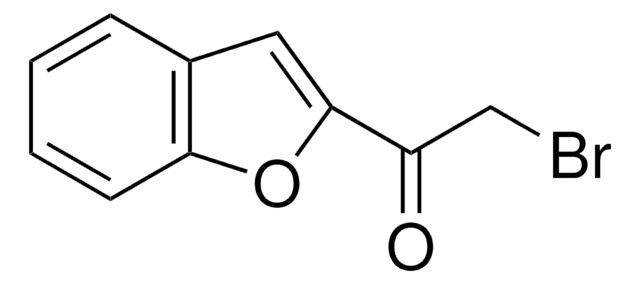
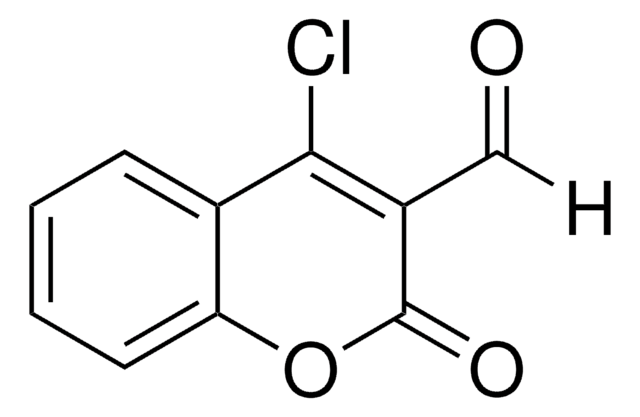
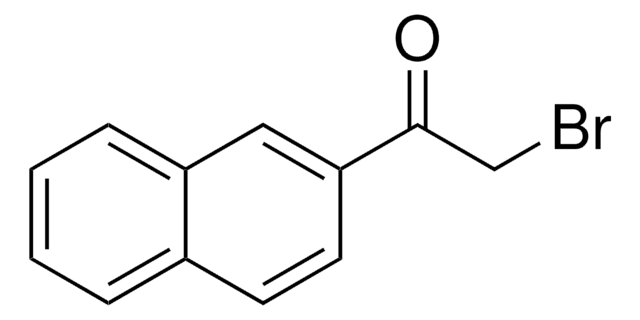
3-(Bromoacetyl)coumarin
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C11H7BrO3
Número de CAS:
Peso molecular:
267.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
solid
mp
164-168 °C (lit.)
functional group
bromo
ester
ketone
SMILES string
BrCC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C11H7BrO3/c12-6-9(13)8-5-7-3-1-2-4-10(7)15-11(8)14/h1-5H,6H2
InChI key
NTYOLVNSXVYRTJ-UHFFFAOYSA-N
Categorías relacionadas
General description
3-(Bromoacetyl)coumarin can be synthesized via the bromination of 3-acetylcoumarin in chloroform.
Application
3-(bromoacetyl)coumarin may be used in the synthesis of the following:
- 3-(bromoacetyl)coumarin oxime via reaction with hydroxylamine hydrochloride in methanol
- 3-(bromoacetyl)coumarin-O-methyloxime via reaction with O-benzylhydroxylammonium chloride/diluted HCl in methanol
- 3-(bromoacetyl)coumarin-O-benzyloxime via reaction with O-benzyl hydroxylamine hydrochloride in methanol
- 3-[2′-(2′′-arylidenehydrazinyl)thiazolyl]coumarins via reaction with benzaldehyde thiosemicarbazones
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
"Synthesis and Antibacterial Activity of Quinolone-Based Compounds Containing a Coumarin Moiety"
Emami S, et al.
Arch. Pharm. (Weinheim), 341(01), 42-48 (2008)
"Synthesis and Oral Hypoglycemic Activity of 3-[5'-Methyl-2'-aryl-3'-(thiazol-2"-yl amino) thiazolidin-4'-one] coumarin Derivatives"
Kini D and Ghate M
Journal of Chemistry, 8(01), 386- 390 (2011)
Saman Khan et al.
Chemico-biological interactions, 290, 64-76 (2018-05-29)
Coumarin is an important bioactive pharmacophore. It is found in plants as a secondary metabolite and exhibits diverse pharmacological properties including anticancer effects against different malignancies. Therapeutic efficacy of coumarin derivatives depends on the pattern of substitution and conjugation with
Ewa Poboży et al.
Mikrochimica acta, 172(3-4), 409-417 (2011-04-08)
Perfluorinated carboxylic acids (PFCAs) represent an important group of persistent perfluorinated organic compounds commonly determined in environmental and biological samples. A reversed-phase HPLC method was developed based on derivatization of the PFCAs with the commercially available fluorescent reagent 3-bromoacetyl coumarin.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico