223670
Bis(cyclopentadienyl)zirconium(IV) chloride hydride
95%
Sinónimos:
Di(cyclopentadienyl)zirconium(IV) chloride hydride, Schwartz’ reagent, Zirconocene chloride hydride
About This Item
Productos recomendados
assay
95%
form
powder
reaction suitability
core: zirconium
reagent type: catalyst
reagent type: reductant
SMILES string
[H][Zr]Cl.[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2
InChI
1S/2C5H5.ClH.Zr.H/c2*1-2-4-5-3-1;;;/h2*1-5H;1H;;/q;;;+1;/p-1
InChI key
PSYHEBZUPSHSKK-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- To convert amides to aldehydes.
- As a reagent for the functionalization of olefins and alkynes.
- As a reducing agent in the preparation of (E)-vinyl organochalcogenides.
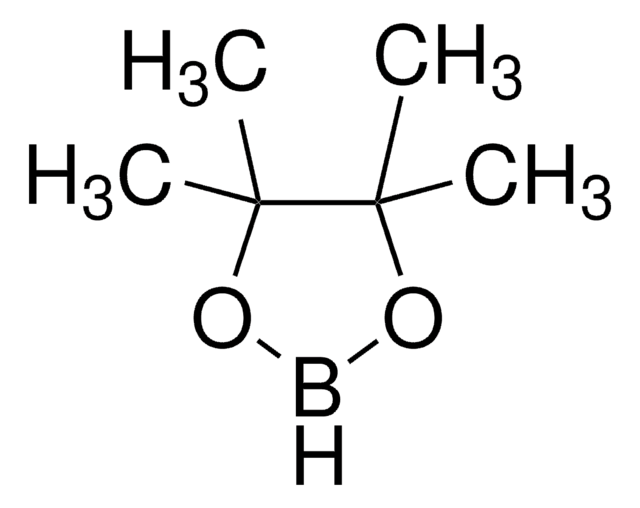
- To prepare a dioxaborolane derivative, which is a key intermediate for the synthesis of bridged-nicotine analogs.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B - Water-react 2
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico