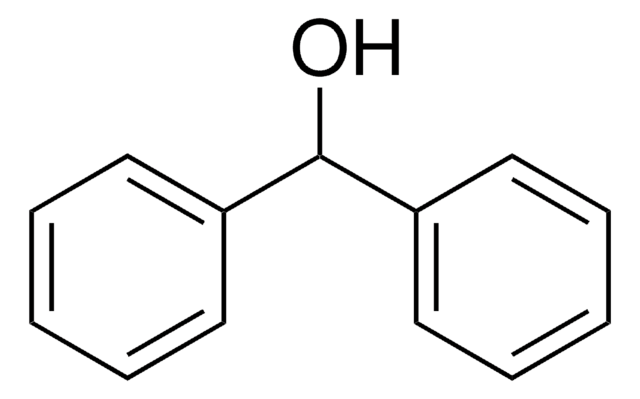
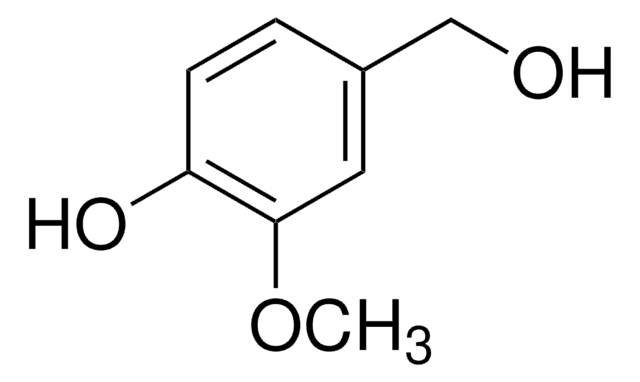
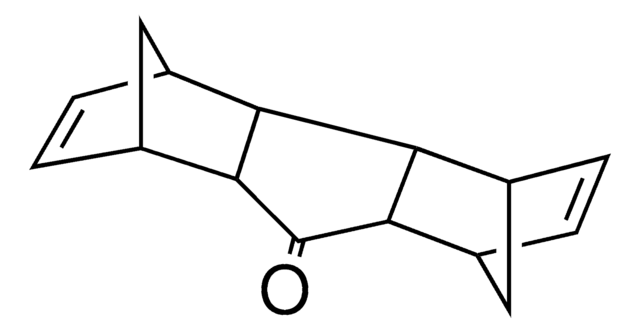
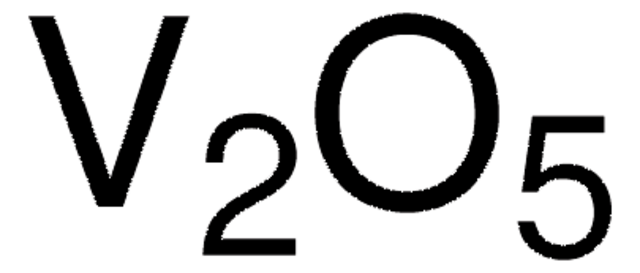213462
Borohidruro de sodio
ReagentPlus®, 99%
Sinónimos:
Tetrahidruroborato de sodio
About This Item
Productos recomendados
Quality Level
product line
ReagentPlus®
assay
99%
form
powder
reaction suitability
reagent type: reductant
mp
>300 °C (dec.) (lit.)
SMILES string
[BH4-].[Na+]
InChI
1S/BH4.Na/h1H4;/q-1;+1
InChI key
YOQDYZUWIQVZSF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Synthesis of cobalt (II, III) oxide (Co3O4) from cobalt chloride (CoCl2) via a co-reduction process.
- Solvent-free reduction of aldehydes and ketones to alcohols in the presence of solid acid as an activator.
- Reductive amination of carbonyl compounds to secondary amines in the presence of silica-gel-supported sulfuric acid as a catalyst.
- Raney nickel-catalyzed reduction of aromatic nitro compounds to aromatic amines.
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B - Water-react 1
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| S957879-1EA | |
| 213462-500G | 4061833498743 |
| 213462-25G | 4061838772862 |
| 213462-100G | 4061838772855 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico