47168
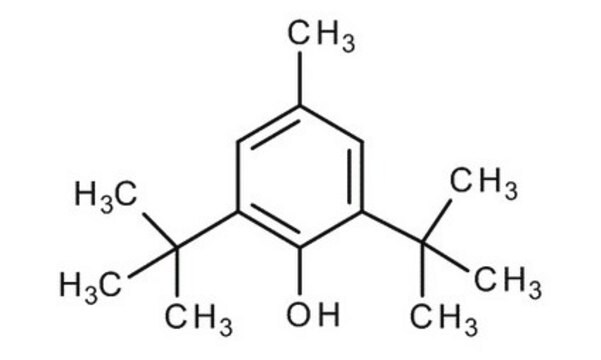
3,5-Di-tert-butyl-4-hydroxytoluene
analytical standard
Synonym(s):
3,5-Di-tert-butyl-4-hydroxytoluene, E321
About This Item
Recommended Products
grade
analytical standard
CofA
current certificate can be downloaded
packaging
pkg of 500 mg
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
storage temp.
room temp
SMILES string
Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3
InChI key
NLZUEZXRPGMBCV-UHFFFAOYSA-N
Gene Information
human ... CAPN1(823)
rat ... Capn1(29153) , Nos1(24598)
General description
Application
Recommended products
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
260.6 °F - open cup
Flash Point(C)
127 °C - open cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
HPLC Analysis of Phenolic Antioxidants on Ascentis® Express C18 2.7 μm
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service