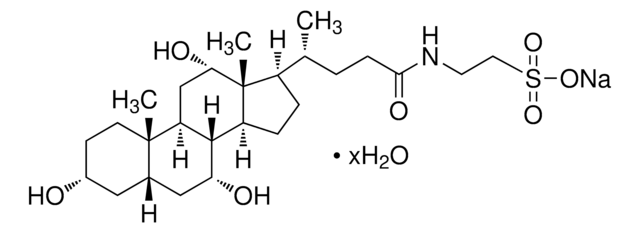
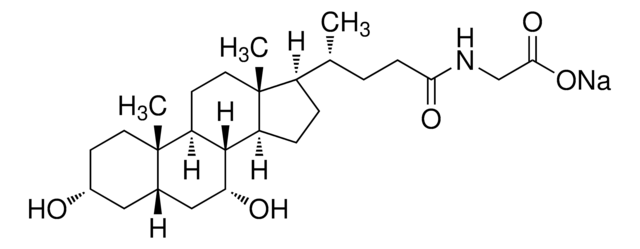
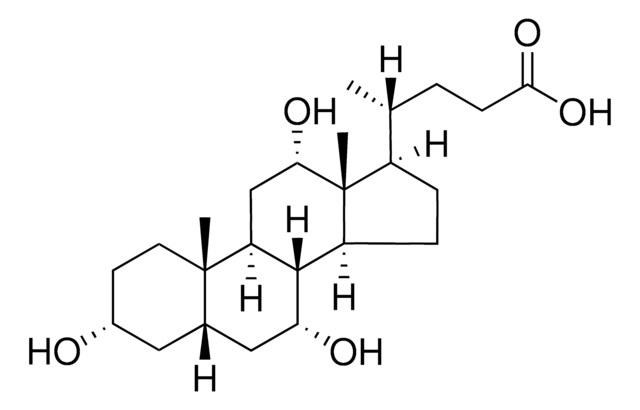
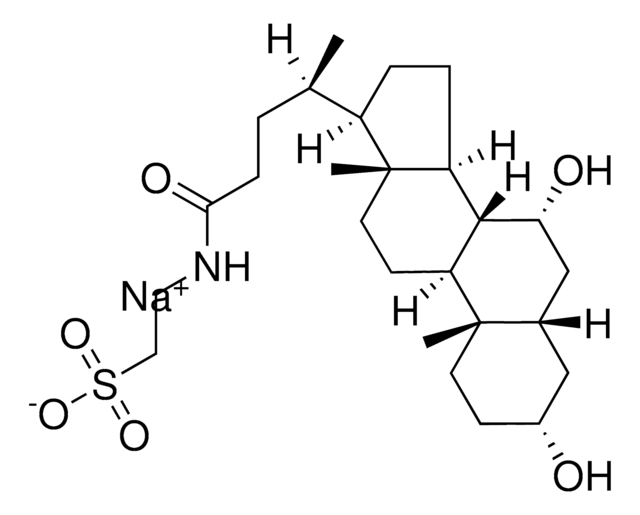
G2878
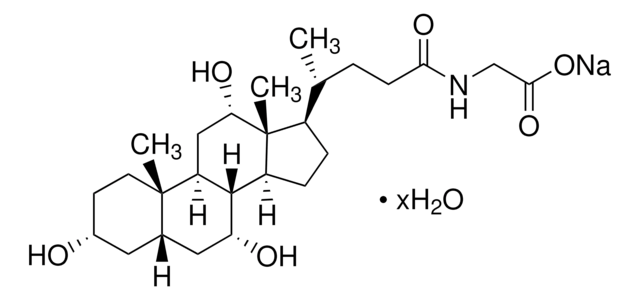
Glycocholic acid hydrate
synthetic, ≥97% (HPLC)
Synonym(s):
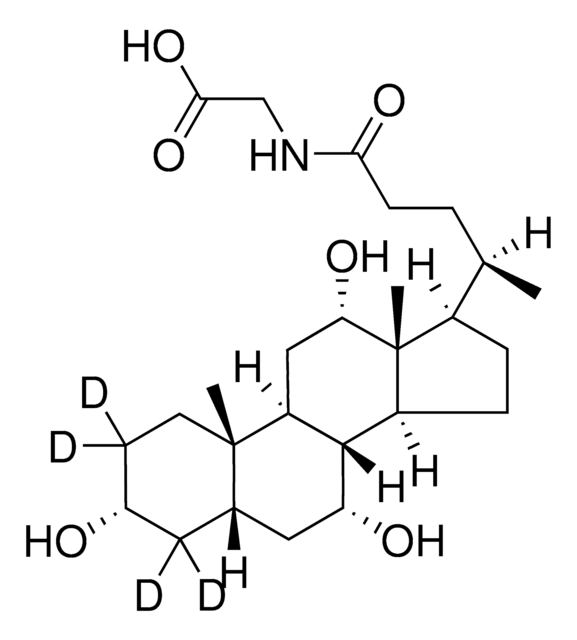
3α,7α,12α-Trihydroxy-5β-cholan-24-oic acid N-(carboxymethyl)amide, Cholylglycine, N-(3α,7α,12α-Trihydroxy-24-oxocholan-24-yl)-glycine
About This Item
Recommended Products
biological source
synthetic
Quality Level
description
anionic
Assay
≥97% (HPLC)
form
powder
mol wt
average mol wt 1000
aggregation number
2.1
technique(s)
enzyme immunoassay: suitable
CMC
7.1
functional group
amide
shipped in
ambient
storage temp.
room temp
SMILES string
O.C[C@H](CCC(=O)NCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C
InChI
1S/C26H43NO6.H2O/c1-14(4-7-22(31)27-13-23(32)33)17-5-6-18-24-19(12-21(30)26(17,18)3)25(2)9-8-16(28)10-15(25)11-20(24)29;/h14-21,24,28-30H,4-13H2,1-3H3,(H,27,31)(H,32,33);1H2/t14-,15+,16-,17-,18+,19+,20-,21+,24+,25+,26-;/m1./s1
InChI key
WDKPRHOCWKLQPK-ZUHYDKSRSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
This method is particularly useful in research into the role of individual bile acids as signaling molecules; suitable for clinical laboratories to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
Related Content
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service