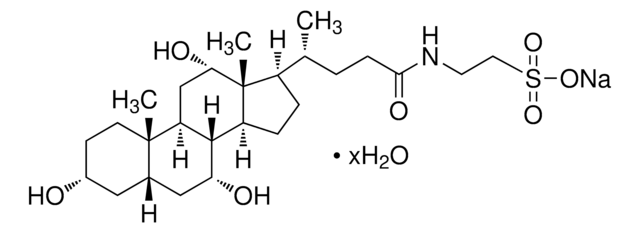
T4009
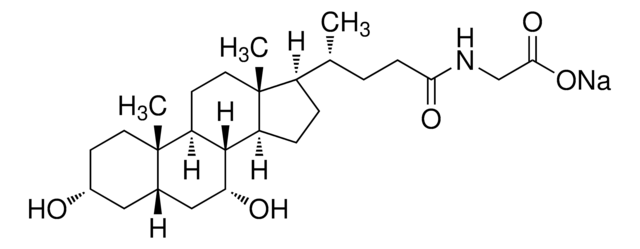
Taurocholic acid sodium salt hydrate
≥95% (HPLC)
Synonym(s):
2-[(3α,7α,12α-Trihydroxy-24-oxo-5β-cholan-24-yl)amino]ethanesulfonic acid, 3α,7α,12α-Trihydroxy-5β-cholan-24-oic acid N-(2-sulfoethyl)amide, Sodium taurocholate hydrate
About This Item
Recommended Products
biological source
synthetic (organic)
description
anionic
Assay
≥95% (HPLC)
form
powder
mol wt
micellar avg mol wt 2100
aggregation number
4
CMC
3-11 mM (20-25°C)
functional group
sulfonic acid
shipped in
ambient
storage temp.
room temp
SMILES string
[Na+].[H]O[H].[H][C@@]12C[C@H](O)CC[C@]1(C)[C@@]3([H])C[C@H](O)[C@]4(C)[C@H](CC[C@@]4([H])[C@]3([H])[C@H](O)C2)[C@H](C)CCC(=O)NCCS([O-])(=O)=O
InChI
1S/C26H45NO7S.Na.H2O/c1-15(4-7-23(31)27-10-11-35(32,33)34)18-5-6-19-24-20(14-22(30)26(18,19)3)25(2)9-8-17(28)12-16(25)13-21(24)29;;/h15-22,24,28-30H,4-14H2,1-3H3,(H,27,31)(H,32,33,34);;1H2/q;+1;/p-1/t15-,16+,17-,18-,19+,20+,21-,22+,24+,25+,26-;;/m1../s1
InChI key
RDAJAQDLEFHVNR-NEMAEHQESA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Preparation Note
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Today, diverse studies report the benefits of probiotics, such as inhibitory effects on pathogens, aid in the management or prevention of chronic intestinal inflammatory diseases or atopic syndromes, and support to the immune system. Potential beneficial applications abound, researchers continue to evaluate the effictiveness and clarify the mechanisms of action of probiotics.
Protocols
This method is particularly useful in research into the role of individual bile acids as signaling molecules; suitable for clinical laboratories to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
Related Content
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service