SBR00070
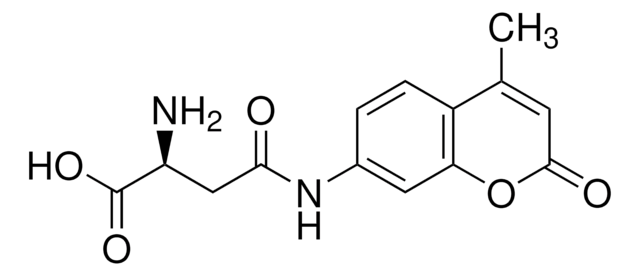
Coumarin Labeled D-Lysine
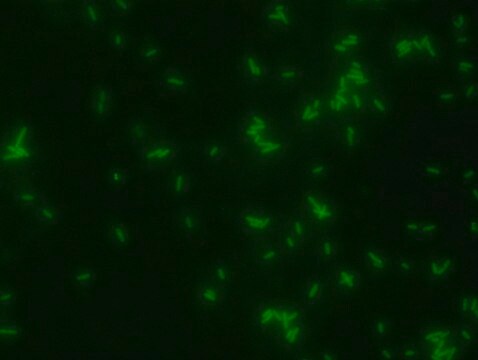
Suitable for fluorescent microbial imaging
Synonym(s):
D-Lysine blue, D-Lysine, N6-[(7-hydroxy-2-oxo-2H-1-benzopyran-3-yl)carbonyl]- (ACI), FDAA
About This Item
Recommended Products
Quality Level
form
solid
storage temp.
−20°C
SMILES string
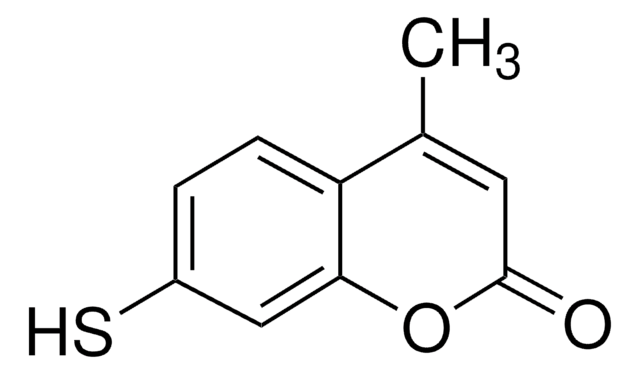
O=C(NCCCC[C@@H](N)C(O)=O)C1=CC2=CC=C(O)C=C2OC1=O
InChI
1S/C16H18N2O6/c17-12(15(21)22)3-1-2-6-18-14(20)11-7-9-4-5-10(19)8-13(9)24-16(11)23/h4-5,7-8,12,19H,1-3,6,17H2,(H,18,20)(H,21,22)/t12-/m1/s1
InChI key
QYOUXTJCRWPQLR-GFCCVEGCSA-N
General description
Application
- Bacterial cell wall morphology
- Bacterial cell wall formation or remodeling activity
- Bacterial viability/activity
- Identify bacterial activity on surfaces or in substances
- Differentiation between various bacterial strains according to their incorporation profile of different D amino acids and sugars
Analysis Note
- Fluorescent microscopyapplication: Coumarin Labeled D-lysine has excitation/emission wavelength rangeat 360/450 nm.
- The recommended working concentrationin fluorescent microscopy imaging application is between 250µM-500 µM inworking medium
- Aliquots of the DMSO solution can bestored at -20 ⁰C, protected from light for at least one month.
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service