S2563
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp.
Synonym(s):
SCDase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
foreign activity
exoglycosidases: α- and β-galactosidase, α- and β-N-acetylgalactosaminidase, β-N-acetylglucosaminidase, α-mannosidase, α-fucosidase, and sialidase., essentially free
protease and sphingomyelinase, essentially free
Quality Level
shipped in
dry ice
storage temp.
−20°C
General description
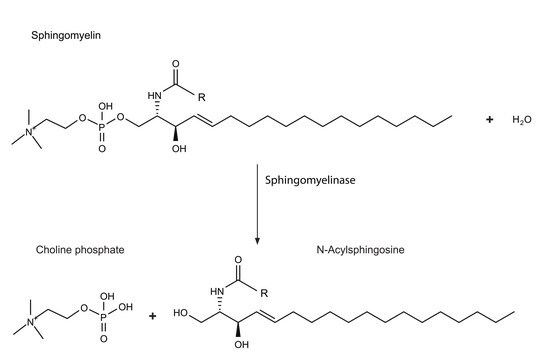
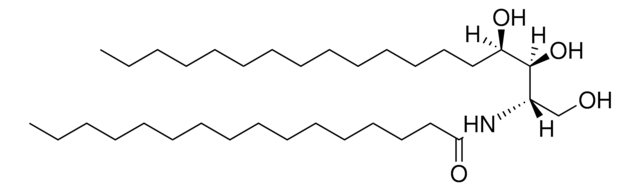
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp. is a hydrolytic enzyme.
Application
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp. has been used in enzymatic conversion and derivatization of sulfatides. It has also been used to hydrolyze the fatty acid chain of mactosyl ceramide.
Biochem/physiol Actions
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp., under certain conditions, can recyclate lyso-sphingolipids. It condenses fatty acids to sphingosine to generate ceramide. The enzyme mainly acts on neutral and acidic glycosphingolipids.
Hydrolyzes the N-acyl linkage between fatty acids and sphingosines in ceramides of various sphingolipids.
Unit Definition
One unit will hydrolyze 1 μmol of asialo GM1 per minute at pH 6.0 at 37 °C.
Physical form
Solution in 50 mM sodium acetate, pH 6.0, with 0.1% Lubrol PX.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Characterization of the reversible nature of the reaction catalyzed by sphingolipid ceramide N-deacylase. A novel form of reverse hydrolysis reaction.
Kita K
European Journal of Biochemistry, 268(3), 592-602 (2001)
Sulfatide Analysis by Mass Spectrometry for Screening of Metachromatic Leukodystrophy in Dried Blood and Urine Samples.
Spacil Z
Clinical Chemistry, 62(1), 279-286 (2016)
A novel enzyme that cleaves the N-acyl linkage of ceramides in various glycosphingolipids as well as sphingomyelin to produce their lyso forms.
Ito M
The Journal of Biological Chemistry, 270(41), 24370-24374 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service