NA0410
GenElute™ HP Endotoxin-Free Plasmid Maxiprep Kit
sufficient for 25 preparations
Synonym(s):
Gen Elute, GenElute Endotoxin-Free Plasmid Kit
About This Item
Recommended Products
usage
sufficient for 25 preparations
Quality Level
greener alternative product characteristics
Designing Safer Chemicals
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
technique(s)
DNA extraction: suitable
greener alternative category
storage temp.
15-25°C
Looking for similar products? Visit Product Comparison Guide
General description
High-quality, endotoxin-free DNA is ready for immediate use for the most demanding applications including transfection with endotoxin-sensitive cells.
Application
Features and Benefits
- Up to 1.2 mg of high-copy plasmid DNA with endotoxin levels of ≤0.1 EU/μg
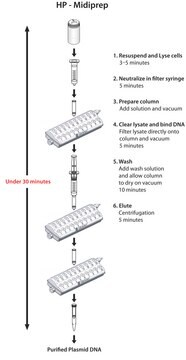
- Only 35 minutes from pelleted cells to purified plasmid
- Convenient vacuum format
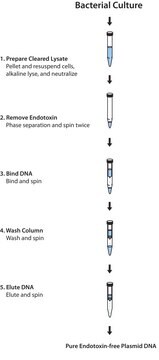
Principle
Other Notes
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Met. Corr. 1 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
Flash Point(F)
77.0 °F - closed cup
Flash Point(C)
25 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Perform rapid isolation of endotoxin-free plasmid DNA using a streamlined procedure that can be completed in as fast as 35 minutes. The GenElute HP Endotoxin-Free Plasmid Maxiprep Kit delivers high plasmid recoveries with levels of endotoxin that are consistently below the industry standard of 0.1 EU/µg.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service