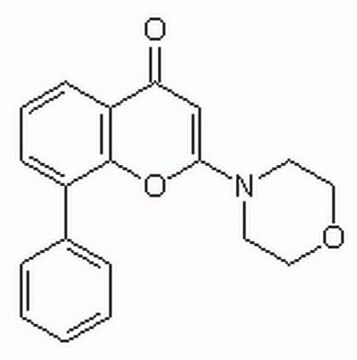
D1692
GW4869
≥90% (NMR), powder, N-SMase inhibitor
Synonym(s):
N,N′-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-3,3′-p-phenylene-bis-acrylamide dihydrochloride
About This Item
Recommended Products
product name
GW4869, ≥90% (NMR)
Quality Level
Assay
≥90% (NMR)
form
powder
storage condition
desiccated
protect from light
color
light yellow to yellow
mp
>300 °C
solubility
DMSO: 0.2 mg/mL
originator
GlaxoSmithKline
storage temp.
2-8°C
SMILES string
Cl.Cl.O=C(Nc1ccc(cc1)C2=NCCN2)\C=C/c3ccc(\C=C/C(=O)Nc4ccc(cc4)C5=NCCN5)cc3
InChI
1S/C30H28N6O2.2ClH/c37-27(35-25-11-7-23(8-12-25)29-31-17-18-32-29)15-5-21-1-2-22(4-3-21)6-16-28(38)36-26-13-9-24(10-14-26)30-33-19-20-34-30;;/h1-16H,17-20H2,(H,31,32)(H,33,34)(H,35,37)(H,36,38);2*1H/b15-5-,16-6-;;
InChI key
NSFKAZDTKIKLKT-LOLTXFFGSA-N
General description
Application
- as an inhibitor of neutral sphingomyelinase and exosome biogenesis
- to analyse the effects of arsenic trioxide (ATO) treatment for hepatoma carcinoma HCCLM3 cells on ceramide production
- to determine the contributions of p75 neurotrophin receptor (p75NTR) and tropomyosin receptor kinase A (TrkA)- coupled pathways to nerve growth factor (NGF)-induced thermal hypersensitivity in rats
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Discover Bioactive Small Molecules for Lipid Signaling Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service