444241
MMP-2/MMP-9 Inhibitor I
The MMP-2/MMP-9 Inhibitor I, also referenced under CAS 193807-58-8, controls the biological activity of MMP-2/MMP-9. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
Synonym(s):
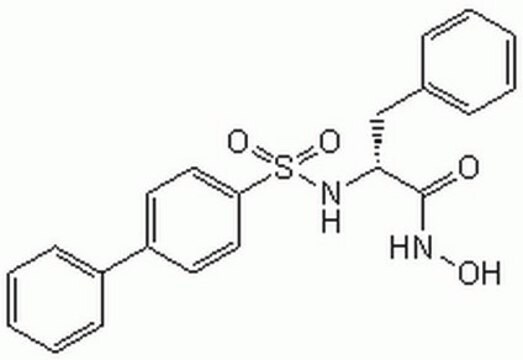
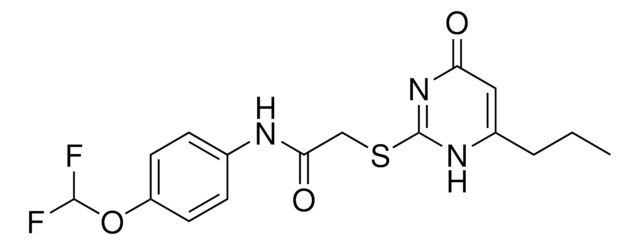
MMP-2/MMP-9 Inhibitor I, (2R)-2-[(4-Biphenylylsulfonyl)amino]-3-phenylpropionic Acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C21H19NO4S
CAS Number:
Molecular Weight:
381.44
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
color
white
solubility
DMSO: 200 mg/mL
shipped in
ambient
storage temp.
−20°C
General description
A potent inhibitor of MMP-2 (IC50 = 310 nM) and MMP-9 (IC50 = 240 nM). Orally active in animal models of tumor growth and metastasis.
A potent inhibitor of MMP-2 (gelatinase A; IC50 = 310 nM) and MMP-9 (gelatinase B; IC50 = 240 nM).
Biochem/physiol Actions
Cell permeable: no
Primary Target
MMP-2, MMP-9
MMP-2, MMP-9
Product does not compete with ATP.
Reversible: no
Target IC50: 310 nM and 240 nM against MMP-2 and MMP-9
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Other Notes
Tamura, Y., et al. 1998. J. Med. Chem.41, 640.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y Tamura et al.
Journal of medicinal chemistry, 41(4), 640-649 (1998-03-04)
Various N-sulfonylamino acid derivatives were synthesized and evaluated for their in vitro and in vivo activities to inhibit type IV collagenase (MMP-9 and MMP-2). When the amino acid residue and the sulfonamide moiety were modified, their inhibitory activities were greatly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service