P-063
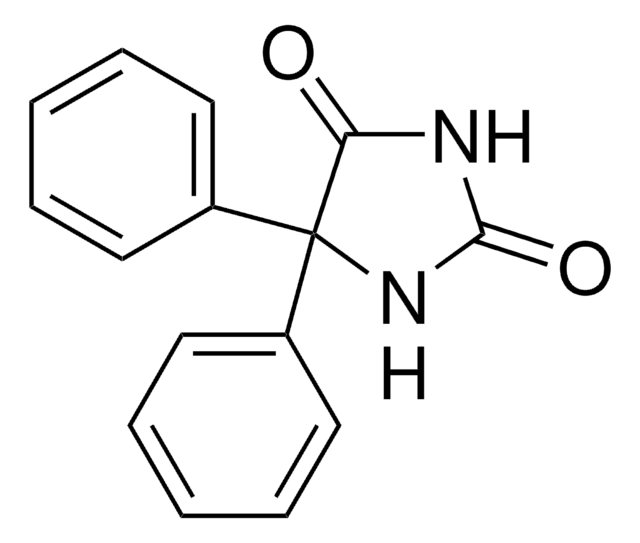
Phenytoin solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
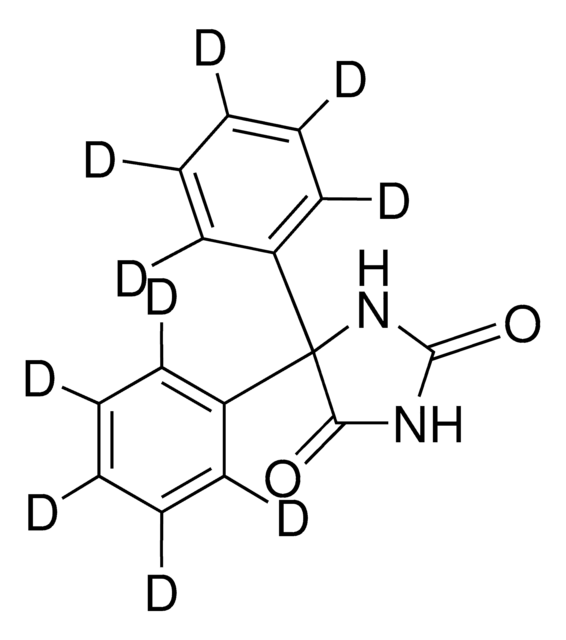
SMILES string
O=C1NC(=O)C(N1)(c2ccccc2)c3ccccc3
InChI
1S/C15H12N2O2/c18-13-15(17-14(19)16-13,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H2,16,17,18,19)
InChI key
CXOFVDLJLONNDW-UHFFFAOYSA-N
Gene Information
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
General description
Application
- Advanced Separation and Extraction of Anticonvulsants: Phenytoin solution is utilized in the fabrication of surface molecularly imprinted polymer membranes. This innovative method enhances the separation and extraction of phenytoin, phenobarbital, and lamotrigine, significantly improving the precision and efficiency of drug monitoring in clinical and research settings (Zhao et al., 2024).
- LC-MS/MS Quantification for Drug Delivery: A sensitive LC-MS/MS method has been developed for the quantification of phenytoin and its major metabolite. This method is applied to both intravenous and intranasal delivery systems, enhancing our understanding of the pharmacokinetics and effectiveness of phenytoin as an anticonvulsant medication (Prentice et al., 2022).
- Clinical Decision Support for Pharmacogenomics: Phenytoin is involved in the appraisal and development of evidence-based clinical decision support systems. This research integrates pharmacogenomic data to optimize perioperative drug use, providing tailored therapeutic strategies based on genetic profiles, particularly for managing epilepsy treatments (Borden et al., 2021).
- Electromembrane Extraction Technique: The development of an electromembrane extraction method followed by capillary electrophoresis for phenytoin determination in exhaled breath condensate. This technique offers a non-invasive approach for therapeutic drug monitoring, critical for ensuring effective seizure control in epilepsy patients (Seyfinejad et al., 2020).
- Drug Crystallization Inhibition: Research into the use of polymer nanogels as reservoirs for phenytoin has shown potential in inhibiting drug crystallization. This approach could revolutionize drug delivery systems by maintaining the stability and bioavailability of phenytoin, enhancing its therapeutic efficacy for treating neurological disorders (Li et al., 2019).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service